Home /
Expert Answers /
Chemistry /
for-each-of-the-following-reactions-calculate-hrxn-srxn-and-grxn-at-25-pa574
(Solved): For each of the following reactions, calculate Hrxn,Srxn, and Grxn at 25 ...
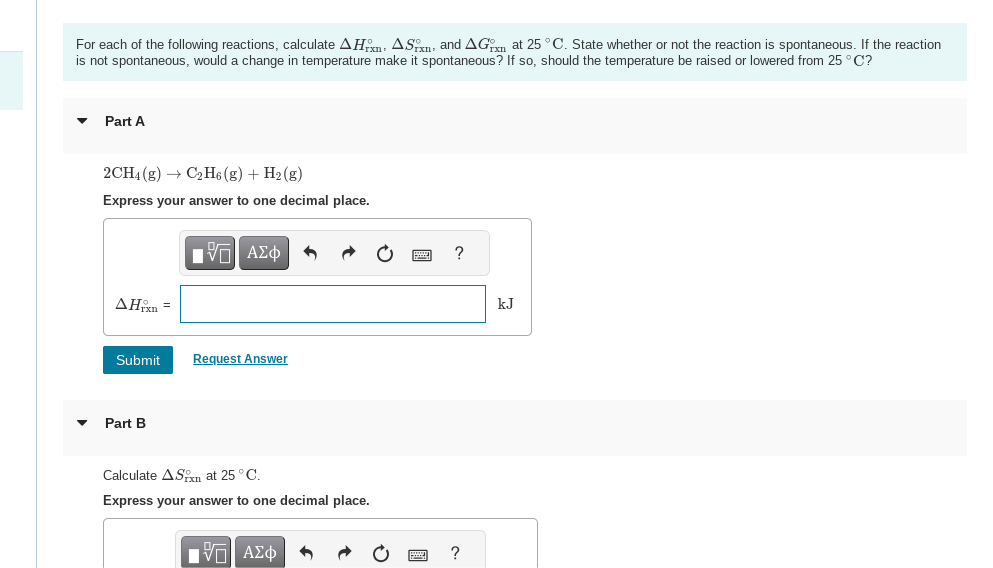
For each of the following reactions, calculate , and at . State whether or not the reaction is spontaneous. If the reaction is not spontaneous, would a change in temperature make it spontaneous? If so, should the temperature be raised or lowered from ? Part A Express your answer to one decimal place. Part B Calculate at . Express your answer to one decimal place.
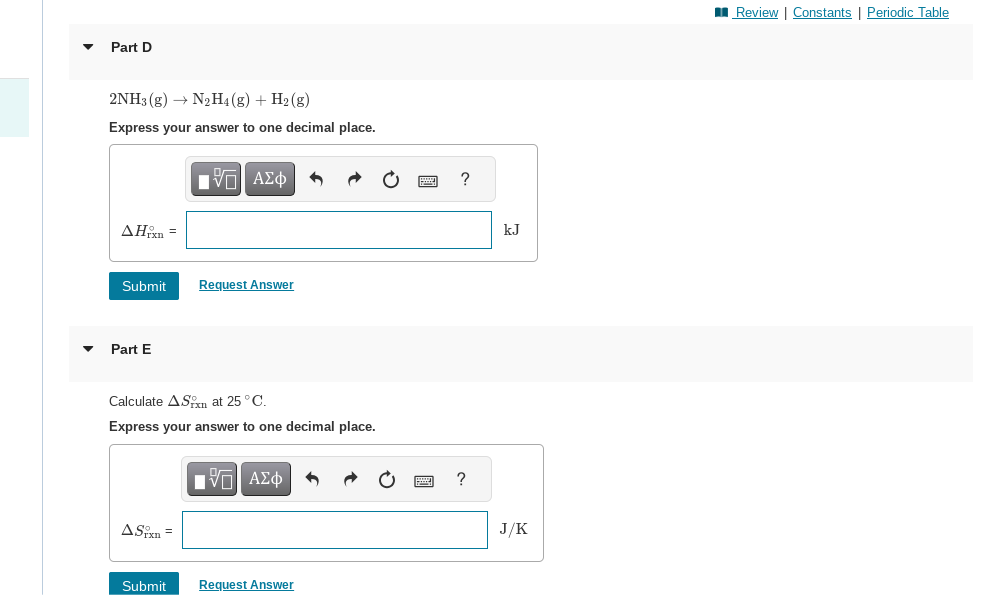
Express your answer to one decimal place. Part E Calculate at . Express your answer to one decimal place.