Home /
Expert Answers /
Chemistry /
for-each-atom-in-the-table-below-write-down-the-subshell-from-which-an-electron-would-have-to-be-r-pa959
(Solved): For each atom in the table below, write down the subshell from which an electron would have to be r ...
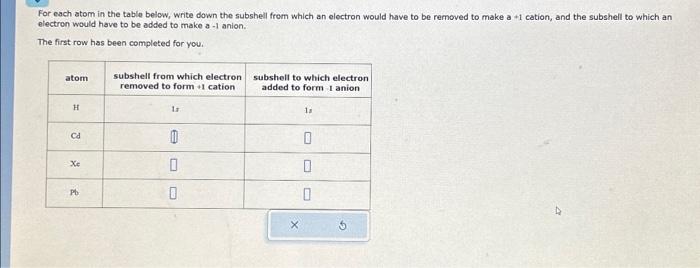
For each atom in the table below, write down the subshell from which an electron would have to be removed to make a +1 cation, and the subshell to which an electron would have to be added to make a -1 anion. The first row has been completed for you.
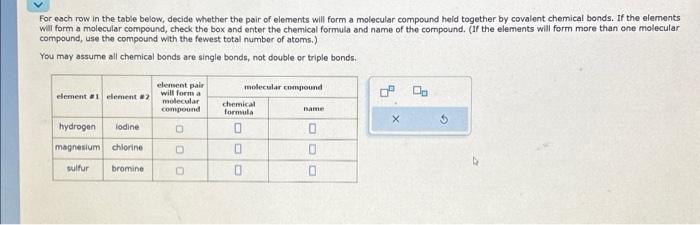
For each row in the table below, decide whether the pair of elements will form a molecular compound held together by covalent chemical bonds. If the elements will form a molecular compound, check the box and enter the chemical formula and name of the compound. (II the elements will form more than one molecular compound, use the compound with the fewest total number of atoms.) You may assume all chemical bonds are single bonds, not double or triple bonds.