Home /
Expert Answers /
Chemistry /
for-a-second-order-reaction-the-rate-constant-k-is-the-slope-of-the-graph-of-a-1-versus-t-b-pa506
(Solved): For a second-order reaction, the rate constant k is the slope of the graph of [A]1 versus t. B ...
![For a second-order reaction, the rate constant \( k \) is the slope of the graph of \( \frac{1}{[\mathrm{~A}]} \) versus \( t](https://media.cheggcdn.com/study/4c9/4c9cf878-881f-4a8b-9205-0a0eaf0dcb90/image)
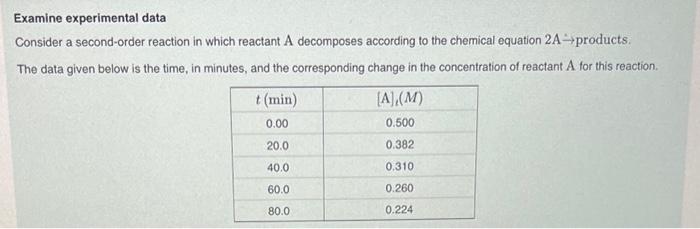
For a second-order reaction, the rate constant is the slope of the graph of versus . Based on this information and the data given, calculate the rate constant for the reaction. Express your answer in to three significant figures.
Examine experimental data Consider a second-order reaction in which reactant decomposes according to the chemical equation products. The data given below is the time, in minutes, and the corresponding change in the concentration of reactant A for this reaction.