Home /
Expert Answers /
Chemistry /
findings-7ml-product-nbsp-130-c-boiling-point-nbsp-experiment-3-preparation-of-isoamyl-acetate-pa606
(Solved): Findings: 7ml product 130 C boiling point Experiment 3 Preparation of Isoamyl Acetate ...
Findings:
7ml product
130 C boiling point
Experiment 3 Preparation of Isoamyl Acetate Learning Objectives M prepare a sample of an ester by reaction of an alcohol with a carboxylic acid appreciate how the continuous removal of a by-product can influence reaction equilibrium perform calculations relating to organic syntheses retrieve information on organic compounds from a variety of sources and cite these sources correctly Introduction An alcohol will react with a carboxylic acid to give an ester with a strong acid acting as a catalyst and with water being formed as a by- product. Here we are reacting isoamyl alcohol with acetic acid to form isoamyl acetate with concentrated sulfuric acid acting as the catalyst, i.e. H?SO4 CH,CO,H (CH3)2CHCH,CH,OH = CH,CO,CH,CH,CH(CH3)2 + H,O acetic acid isoamyl alcohol isoamyl acetate In order to force the reaction to the right, and thereby increase the speed of esterification, the water must be continuously removed as it is formed. This is done by condensing it as a layer in the side arm of the apparatus. A little isoamyl alcohol (b.p. 130°C) will also be distilled over, but since this is immiscible with water and also less dense (d = 0.81 g ml-¹), it will form the upper layer. It is essential to perform the reaction under very gentle reflux to minimise the volume of isoamyl alcohol collected, since this is then not available for reaction. When the theoretical amount of water (i.e. the amount that would be formed when the esterification is complete) has been collected then the reaction can be stopped. This collection technique makes use of what is known as a modified Dean and Stark apparatus. +
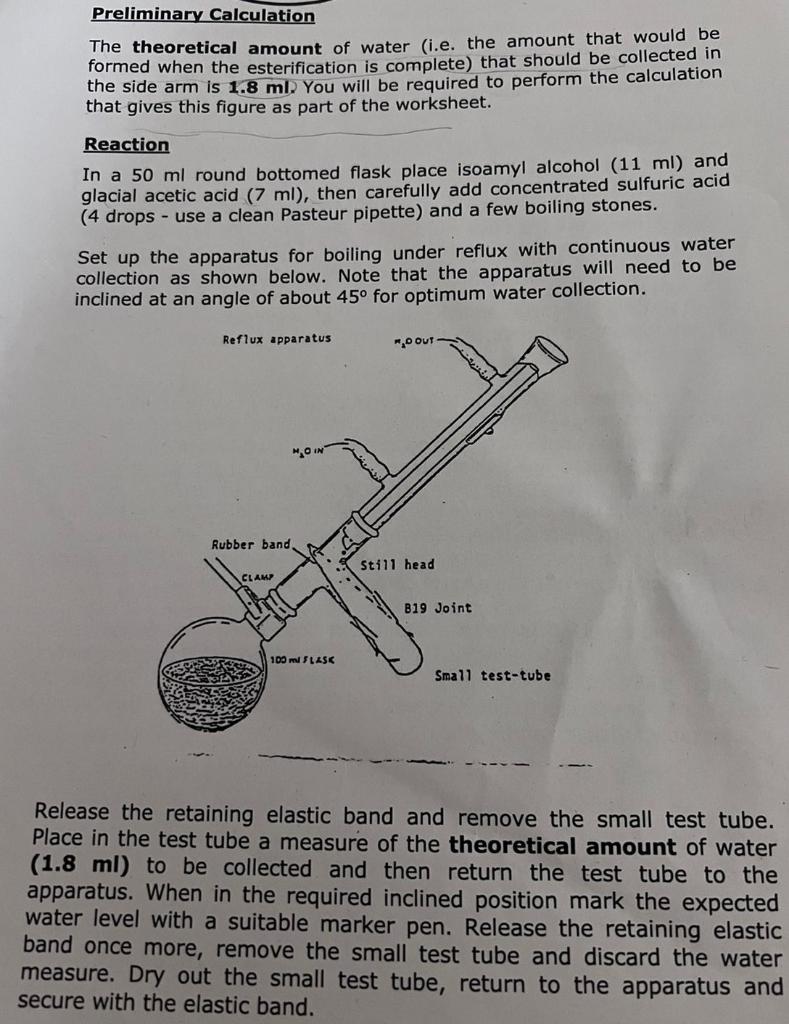
Preliminary Calculation The theoretical amount of water (i.e. the amount that would be formed when the esterification is complete) that should be collected in the side arm is 1.8 ml. You will be required to perform the calculation that gives this figure as part of the worksheet. Reaction In a 50 ml round bottomed flask place isoamyl alcohol (11 ml) and glacial acetic acid (7 ml), then carefully add concentrated sulfuric acid (4 drops - use a clean Pasteur pipette) and a few boiling stones. Set up the apparatus for boiling under reflux with continuous water collection as shown below. Note that the apparatus will need to be inclined at an angle of about 45° for optimum water collection. Reflux apparatus Rubber band. CLAMP 100 ml FLASK O OUT Still head 819 Joint Small test-tube Release the retaining elastic band and remove the small test tube. Place in the test tube a measure of the theoretical amount of water (1.8 ml) to be collected and then return the test tube to the apparatus. When in the required inclined position mark the expected water level with a suitable marker pen. Release the retaining elastic band once more, remove the small test tube and discard the water measure. Dry out the small test tube, return to the apparatus and secure with the elastic band.
Using a low Bunsen burner flame, boil under very gentle reflux. Water will then collect in the side tube and after about 20-30 min should be near to the correct level for complete reaction. At this stage stop heating and allow the assembly to cool, then tip the side tube contents back into the reaction flask. Isolation of Product Pour the contents of the flask into a mixture of crushed ice (10 g) and water (20 ml) contained in a 250 ml beaker. When all of the ice has melted, transfer to a 250 ml separating funnel set-up in the fume cupboard. Rinse out the flask and beaker with ether (25 ml) and add this to the separating funnel. Shake to mix with the separating funnel inverted then release pressure and repeat several times. Run off and discard the lower aqueous layer. Next wash the ether layer with water (25 ml) and run off/discard the lower aqueous layer, then wash the ether layer with aqueous sodium carbonate solution (25 ml) and run off/discard the lower aqueous layer. Run off the ether layer into a 100 ml beaker and dry over a small amount of anhydrous magnesium sulfate, then gravity filter off the drying agent through a funnel containing a small cotton wool plug into another 100 ml beaker containing a few boiling stones. Remove all of the solvent on the steam bath to obtain crude product. NOTE: Diethyl ether (b.p. 35 °C) is highly flammable and must be completely removed before the final distillation is attempted. Thus after the bubbles of diethyl ether emanating from the boiling stones have ceased continue warming on the steam bath for a further 10 minutes to ensure complete solvent removal. Transfer the crude product to a distillation flask containing a few boiling stones and then distil using a low flame, collecting the fraction boiling between 135°C and 145°C in a 10 ml measuring cylinder. Record the volume of pure product collected and the exact boiling point, then submit in a suitably labelled sample vial.
% Theoretical yield = Experimental yield Theoretical yield : x 100 : Appearance of sample Boiling point of sample Literature value of boiling point : Literature reference used Remember to submit your sample in a suitably labelled sealed sample vial. To calculate the theoretical amount of water that will be produced: Scale of reaction .. Number of moles of water that will be produced Molecular weight of water H?C C C-C-OH Isoamyl alcohol H H? x 100 = .. Mass of water that will be produced Density of water :: Volume of water that will be produced = 2 marks = 1 mark % 1 mark 1 mark 1 mark mol mol g mol-1 g = 1.0 g ml-¹ ml 5 marks Part 2 - Questions (i) Isoamyl alcohol (below) is a primary alcohol. Draw structures for three constitutional isomers of isoamyl alcohol that are also primary alcohols. 6 marks CH,
(ii) Hexan-2-ol (below) is a secondary alcohol. Draw structures for five constitutional isomers of hexan-2-ol that are also secondary alcohols. 10 marks OH Hexan-2-ol (b) Draw the structure of the ester that would be obtained from reaction of isoamyl alcohol with p-isopropylbenzoic acid. 6 marks (c) To prepare 25 ml of a 50 mM solution of the ester butyl ethanoate, what volume of butyl ethanoate (in µl) should be dissolved in water? Show all of the calculations used to reach your answer. The density of butyl ethanoate is 0.88 g ml-¹. 16 marks
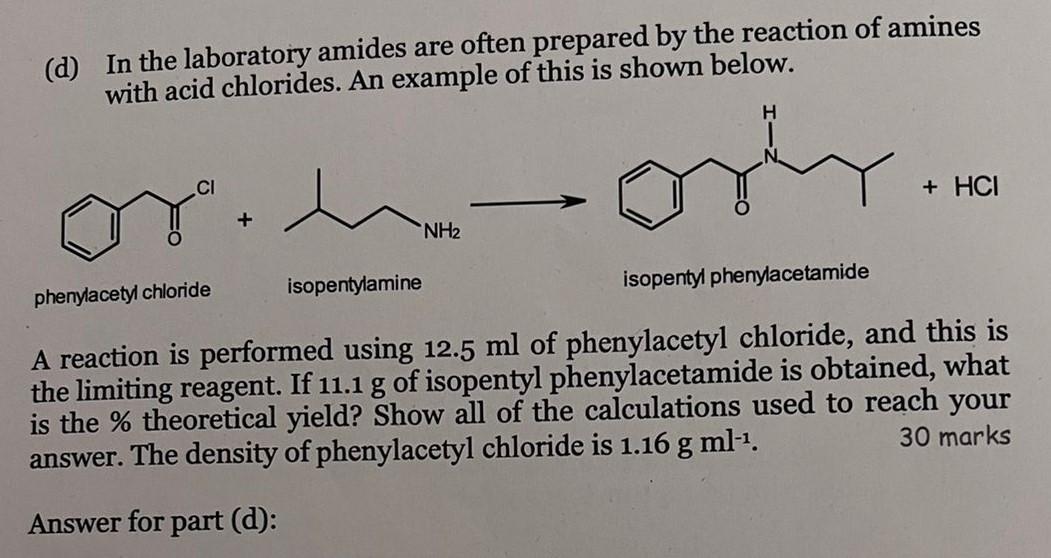
(d) In the laboratory amides are often prepared by the reaction of amines with acid chlorides. An example of this is shown below. or. phenylacetyl chloride + isopentylamine NH? H or + HCI isopentyl phenylacetamide A reaction is performed using 12.5 ml of phenylacetyl chloride, and this is the limiting reagent. If 11.1 g of isopentyl phenylacetamide is obtained, what is the % theoretical yield? Show all of the calculations used to reach your 30 marks answer. The density of phenylacetyl chloride is 1.16 g ml-¹. Answer for part (d):