Home /
Expert Answers /
Chemistry /
find-the-ion-concentration-in-mol-l-of-each-complete-reaction-all-4-use-correct-sig-figs-thank-y-pa809
(Solved): Find the ion concentration in mol/L of each complete reaction. (All 4) Use correct sig figs. thank y ...
Find the ion concentration in mol/L of each complete reaction. (All 4) Use correct sig figs. thank you!!
-the other perosn that solved rhis problem on chegg was wrong!!
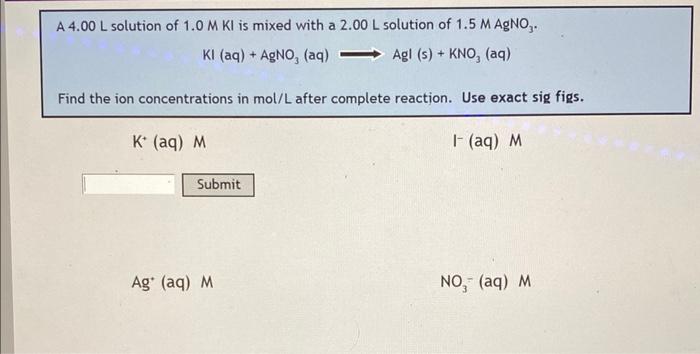

A \( 4.00 \mathrm{~L} \) solution of \( 1.0 \mathrm{M} \mathrm{KI} \) is mixed with a \( 2.00 \mathrm{~L} \) solution of \( 1.5 \mathrm{M} \mathrm{AgNO}_{3} \). \[ \mathrm{KI}(\mathrm{aq})+\mathrm{AgNO}_{3}(\mathrm{aq}) \Longrightarrow \mathrm{AgI}(\mathrm{s})+\mathrm{KNO}_{3}(\mathrm{aq}) \] Find the ion concentrations in mol/L after complete reaction. Use exact sig figs.
Expert Answer
given volume of KI = 4 L molarity = 1 M So # mols of K+ and I- before mixing = volume * molarity = 4 L * 1 M = 4.0 mols volu