Home /
Expert Answers /
Chemistry /
find-molar-heat-of-combustion-conbustion-homb-calorinseter-in-an-experiment-a-0-43978-sample-of-pa161
(Solved): find Molar heat of combustion Conbustion (homb) calorinseter. In an experiment, a 0.43978 sample of ...
find Molar heat of combustion
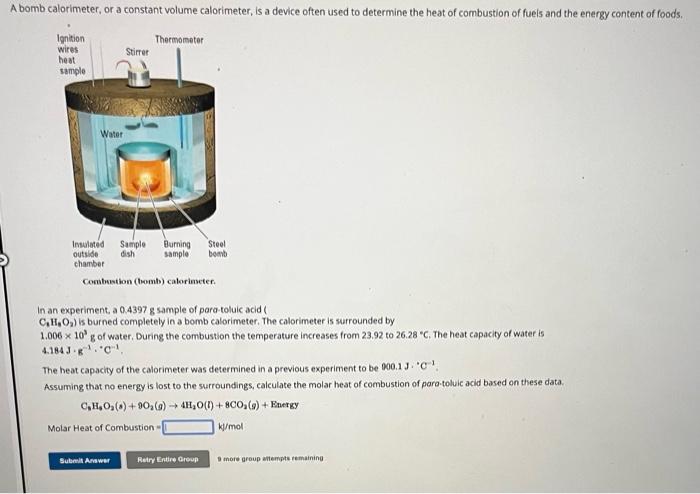

Conbustion (homb) calorinseter. In an experiment, a 0.43978 sample of para-toluic acid ( ) is burned completely in a bomb calorimeter. The calorimeter is surrounded by of water, During the combustion the temperature increases from 23.92 to 26.28 " . The heat capacity of water is , The heat capacity of the calorimeter was determined in a previous experiment to be . Assuming that no energy is lost to the surroundings, calculate the molar heat of combustion of para-toluic acid based on these data. Molar Heat of Combustion = 3 more group entempteremaining