Home /
Expert Answers /
Chemistry /
experiment-1-suppose-a-student-repeats-the-experiment-but-adds-25-mathrm-g-of-sodium-bi-pa760
(Solved): Experiment 1: Suppose a student repeats the experiment, but adds \( 25 \mathrm{~g} \) of sodium bi ...
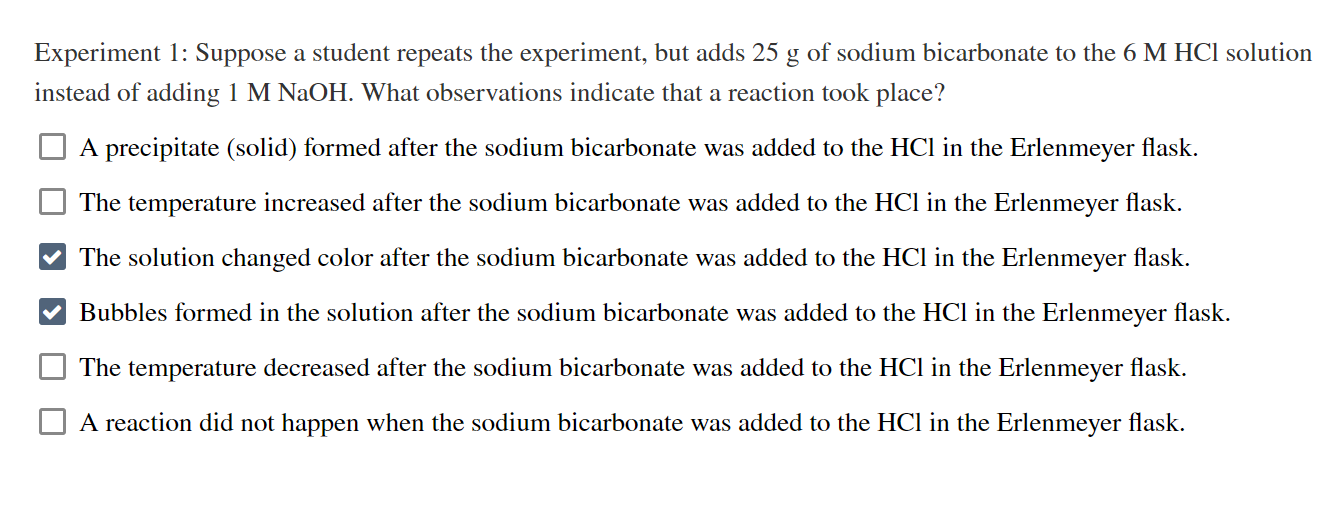
Experiment 1: Suppose a student repeats the experiment, but adds \( 25 \mathrm{~g} \) of sodium bicarbonate to the \( 6 \mathrm{M} \mathrm{HCl} \) solution instead of adding \( 1 \mathrm{M} \mathrm{NaOH} \). What observations indicate that a reaction took place? A precipitate (solid) formed after the sodium bicarbonate was added to the \( \mathrm{HCl} \) in the Erlenmeyer flask. The temperature increased after the sodium bicarbonate was added to the \( \mathrm{HCl} \) in the Erlenmeyer flask. The solution changed color after the sodium bicarbonate was added to the \( \mathrm{HCl} \) in the Erlenmeyer flask. Bubbles formed in the solution after the sodium bicarbonate was added to the \( \mathrm{HCl} \) in the Erlenmeyer flask. The temperature decreased after the sodium bicarbonate was added to the \( \mathrm{HCl} \) in the Erlenmeyer flask. A reaction did not happen when the sodium bicarbonate was added to the \( \mathrm{HCl} \) in the Erlenmeyer flask.
Expert Answer
NaHCO3 (aq) + HCl (aq) ? NaCl (aq) + CO2 (g) + H2O (l) The temperature in