Home /
Expert Answers /
Chemistry /
exercise-8-the-freezing-point-depression-constant-of-cyclohexane-trial-1-94-2569-2271249-report-dat-pa461
(Solved): EXERCISE 8 The Freezing Point Depression Constant of Cyclohexane Trial 1 94.2569 2271249 Report Dat ...
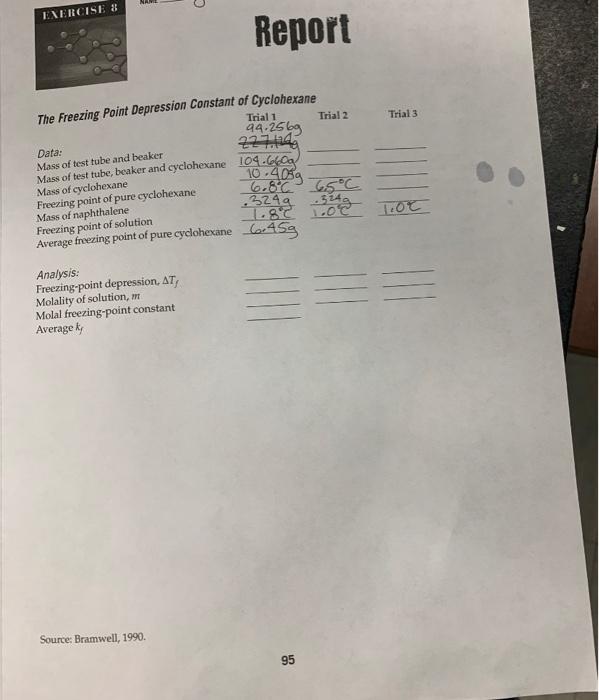
EXERCISE 8 The Freezing Point Depression Constant of Cyclohexane Trial 1 94.2569 2271249 Report Data: Mass of test tube and beaker Mass of test tube, beaker and cyclohexane Mass of cyclohexane Freezing point of pure cyclohexane Mass of naphthalene Freezing point of solution Average freezing point of pure cyclohexane 45g Analysis: Freezing-point depression, AT, Molality of solution, m Molal freezing-point constant Average ky Source: Bramwell, 1990. Trial 2 104.660a 10.405 6.8°C 6.5°C 3249 1.8°C 1.0°C 324 95 ||| Trial 3 T.OC
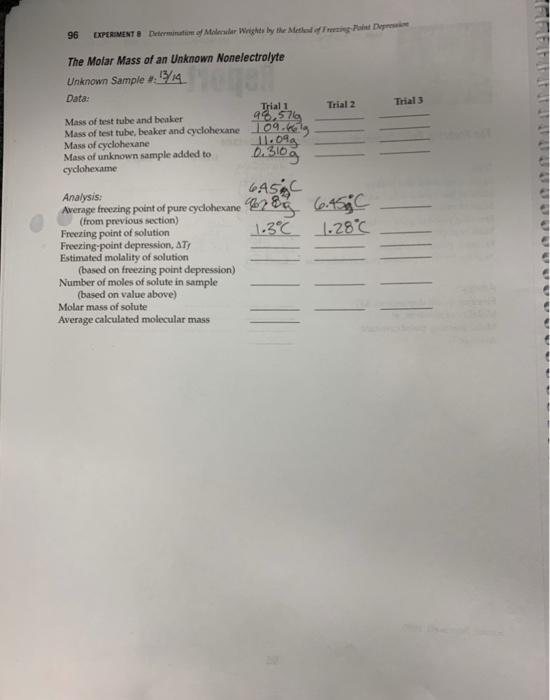
96 EXPERIMENT 8 Determination of Molecular Wrights by the Method of Freeting Point Depression The Molar Mass of an Unknown Nonelectrolyte Unknown Sample #13/14 Mone Data: Trial 1 Mass of test tube and beaker 98,571 Mass of test tube, beaker and cyclohexane 109.klg Mass of cyclohexane Mass of unknown sample added to cyclohexame 11.09 2.310g Analysis: GASC Average freezing point of pure cyclohexane 78 previous Freezing point of solution Freezing-point depression, ATY Estimated molality of solution (based on freezing point depression) Number of moles of solute in sample (based on value above) Molar mass of solute Average calculated molecular mass Trial 2 6.45C 1.3°C 1.28C Trial 3
Expert Answer
Solution: Analysis for trail-1 molality = no of moles of solute / volume of solvent (kg) so that molality = (0.37 / 128.17) / 0.00712 kg = 0.002887 / 0.00712 = 0.405 molal so that \DeltaT = Kb . m Tf(solvent) - Tf(Solu) = Kb . m 4.9 - 1.6 = Kb . (0.4