Home /
Expert Answers /
Physics /
example-4-determine-what-mass-of-steam-initially-at-t-i-text-steam-150-circ-mathrm-c-pa673
(Solved): Example 4: Determine what mass of steam initially at \( T_{i \text { steam }}=150^{\circ} \mathrm{C ...
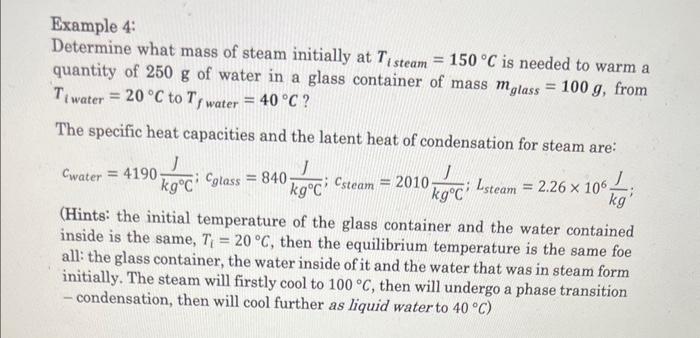
Example 4: Determine what mass of steam initially at \( T_{i \text { steam }}=150^{\circ} \mathrm{C} \) is needed to warm a quantity of \( 250 \mathrm{~g} \) of water in a glass container of mass \( m_{\text {glass }}=100 \mathrm{~g} \), from \( T_{\text {twater }}=20^{\circ} \mathrm{C} \) to \( T_{f \text { water }}=40^{\circ} \mathrm{C} \) ? The specific heat capacities and the latent heat of condensation for steam are: \[ c_{\text {water }}=4190 \frac{\mathrm{J}}{\mathrm{kg}^{\circ} \mathrm{C}} ; c_{\text {glass }}=840 \frac{\mathrm{J}}{\mathrm{kg}^{\circ} \mathrm{C}} ; c_{\text {steam }}=2010 \frac{\mathrm{J}}{\mathrm{kg}^{\circ} \mathrm{C}} ; L_{\text {steam }}=2.26 \times 10^{6} \frac{\mathrm{J}}{\mathrm{kg}} \text {; } \] (Hints: the initial temperature of the glass container and the water contained inside is the same, \( T_{\mathrm{i}}=20^{\circ} \mathrm{C} \), then the equilibrium temperature is the same foe all: the glass container, the water inside of it and the water that was in steam form initially. The steam will firstly cool to \( 100^{\circ} \mathrm{C} \), then will undergo a phase transition - condensation, then will cool further as liquid water to \( 40^{\circ} \mathrm{C} \) )
Expert Answer
Using the energy conservation, He