Home /
Expert Answers /
Chemical Engineering /
example-3-the-pure-component-vapor-pressure-of-acetone-acetonitrile-and-nitromethane-can-by-rep-pa475
(Solved): Example (3): The pure component vapor pressure of Acetone, Acetonitrile and Nitromethane can by rep ...
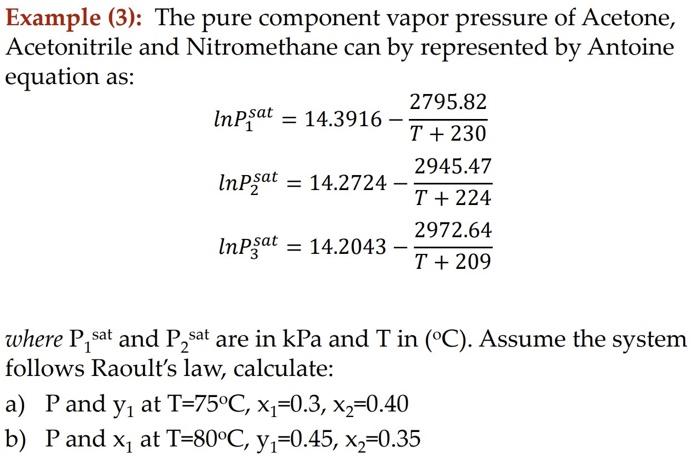
Example (3): The pure component vapor pressure of Acetone, Acetonitrile and Nitromethane can by represented by Antoine equation as: \[ \begin{array}{l} \ln P_{1}^{\text {sat }}=14.3916-\frac{2795.82}{T+230} \\ \ln P_{2}^{\text {sat }}=14.2724-\frac{2945.47}{T+224} \\ \ln P_{3}^{\text {sat }}=14.2043-\frac{2972.64}{T+209} \end{array} \] where \( \mathrm{P}_{1} \) sat and \( \mathrm{P}_{2} \) sat are in \( \mathrm{kPa} \) and \( \mathrm{T} \) in \( \left({ }^{\circ} \mathrm{C}\right) \). Assume the system follows Raoult's law, calculate: a) \( \mathrm{P} \) and \( \mathrm{y}_{1} \) at \( \mathrm{T}=75^{\circ} \mathrm{C}, \mathrm{x}_{1}=0.3, \mathrm{x}_{2}=0.40 \) b) \( \mathrm{P} \) and \( \mathrm{x}_{1} \) at \( \mathrm{T}=80^{\circ} \mathrm{C}, \mathrm{y}_{1}=0.45, \mathrm{x}_{2}=0.35 \)