Home /
Expert Answers /
Chemistry /
example-16-1-identify-the-conjugate-acid-base-pairs-in-the-reaction-between-ammonia-and-hydrofluoric-pa734
(Solved): Example 16.1 Identify the conjugate acid-base pairs in the reaction between ammonia and hydrofluoric ...
Example 16.1 Identify the conjugate acid-base pairs in the reaction between ammonia and hydrofluoric acid in aqueous solution NH3(aq) + HF(aq) = NH+ (aq) + F¯ (aq) Strategy Remember that a conjugate base always has one fewer H atom and one more negative charge (or one fewer positive charge) than the formula of the corresponding acid. Solution NH3 has one fewer H atom and one fewer positive charge than NH. F has one fewer H atom and one more negative charge than HF. Therefore, the conjugate acid-base pairs are (1) NH4 and NH3 and (2) HF and F¯. Practice Exercise Identify the conjugate acid-base pairs for the reaction CN + H?O HCN + OH
PLEASE HELP ME ANSWER THE EXAMPLE AND THE PRACTICE PROBLEMS
![\[
\left.\mathrm{NH}_{3}(a q)+\mathrm{HF}^{(a q}\right)=\mathrm{NH}_{4}^{+}(a q)+\mathrm{F}^{-}(a q)
\]
Srrategy Remember tha](https://media.cheggcdn.com/study/83b/83b7b72f-8914-47d8-93e7-5a9bcbbd152d/image)


![In a \( \mathrm{NaOH} \) solution \( \left[\mathrm{OH}^{-}\right] \)is \( 7.2 \times 10^{-5} \mathrm{M} \). Calculate the \(](https://media.cheggcdn.com/study/4fc/4fc713d7-0f59-45c2-bd7e-ec0ba56c4af2/image)



Srrategy Remember that a conjugate base always has one fewer atom and one more negative charge (or one fewer positive charge) than the formula of the corresponding acid. Solution has one fewer atom and one fewer positive charge than has one fewer atom and one more negative charge than . Therefore, the conjugate acid-base pairs are (1) and and (2) F - Practice Exercise Identify the conjugate acid-base pairs for the reaction
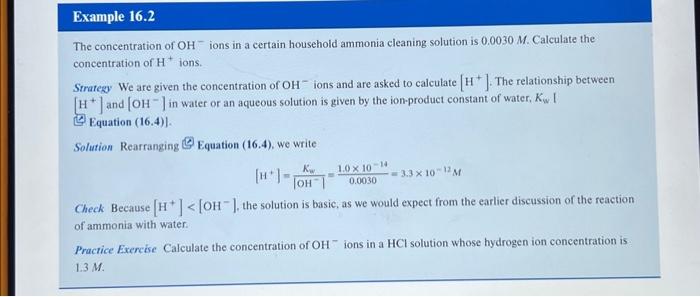
The concentration of - ions in a certain household ammonia cleaning solution is . Calculate the concentration of ions. Strategy We are given the concentration of ions and are asked to calculate . The relationship between and in water or an aqueous solution is given by the ion-product constant of water, ] Equation Solution Rearranging Squation (16.4), we write Check Because , the solution is basic, as we would expect from the earlier discussion of the reaction of ammonis with water. Pracrice Exercise Calculate the concentration of ions in a solution whose hydrogen ion concentration is .
The concentration of ions in a bottle of table wine was right after the cork was removed. Only half of the wine was consumed. The other half, after it had been standing open to the air for a month, was found to have a hydrogen ion concentration equal to . Calculate the of the wine on these two occasions. Srratecy. We are given the ion concentration and are asked to calculate the of the solution. What is the definition of ? Solution According to [?uation (16.5). . When the bottle was first opened, , which we substitute in Equation (16.5): On the second occasion, , so that In esch case, the pH has only two significant figures. The two digits to the right of the decimal in 3.39 teil us that there are fwo significant figures in the original number (see Appendix 3 ) Comment The increase in hydrogen ion concentration (or decrease in ) is largely the result of the conversion of some of the alcohol (ethanol) to acetic acid, a reaction that takes place in the presence of molecular oxygen. Practice Exercise Nitric acid (HNO ) is used in the production of fertilizer, dyes, drugs, and explosives, Caleulate the of a solution having a hydrogen ion concentration of .
The of rainwater collected in a certain region of the northeastern United States on a particular day was 5.23. Calculate the ion concentration of the rainwater. Strategy. Here we are given the of a solution and asked to calculate . Because is defined as , we can solve for by taking the antilog of the ; that is, , as shown in [?) Equation . Solution From Equation (16.5). Therefore, To calculate , we need to take the antilog of -5.23 : Scientific calcilators hove an antilog function that is sometimes labeled iNV log or . Check Because the is between 5 and 6 , we can expect to be between and . Therefore. the answer is reasonable. Practice Exercise The of a certain orange juice is 3.33. Calculate the ion concentration.
In a solution is . Calculate the of the solution. Srrategy Solving this problem takes two steps. First, we need to calculate the pOH using Equation (16.8). Next, we use ? Equation (16.10) to calculate the of the solution. Solution We use [?? Equation (16.8): Now we use [? Equation (16.10) Alternatively, we can use the ion-product constant of water, to calculate , and then we can calculate the from the . Try it. Check The answer shows that the solution is basic , which is consistent with a solution. Pructice Exereise The ion concentration of a blood sample is . What is the of the blood?
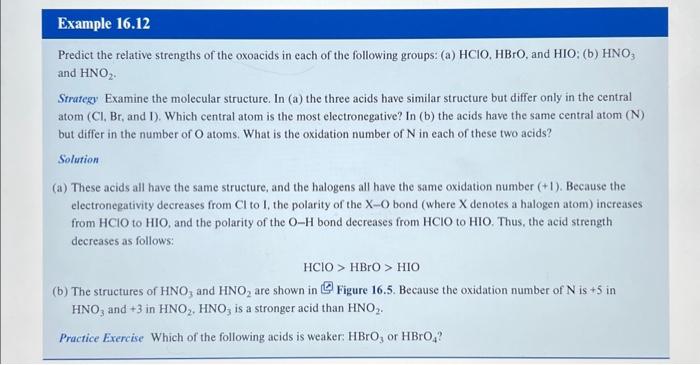
Predict the relative strengths of the oxoacids in each of the following groups: (a) , and : (b) and Strategy Examine the molecular structure. In (a) the three acids bave similar structure but differ only in the central atom ( , and I). Which central atom is the most electronegative? In (b) the acids have the same central atom ( ) but differ in the number of atoms. What is the oxidation number of in each of these two acids? Solution (a) These acids all have the same structure, and the halogens all have the same oxidation number . Because the electronegativity decreases from to , the polarity of the bond (where denotes a halogen atom) increases from to , and the polarity of the bond decreases from to . Thus, the acid strength decreases as follows: (b) The structures of and are shown in Figure 16.5. Because the oxidation number of is +5 in and +3 in is a stronger acid than . Practice Exercise Which of the following acids is weaker: or ?
Predict whether the following solutions will be acidic, basic, or nearly neutral: (a) , (b) , (d) . Strategy In deciding whether a salt will undergo hydrolysis, ask yourself the following questions: Is the cation a highly charged metal ion or an ammonium ion? Is the anion the conjugate base of a weak acid? If yes to either question. then hydrolysis will occur. In cases where both the cation and the anion react with water, the of the solution will depend on the relative magnitudes of for the cation and for the anion (see [? Table 16.7). Solution We first break up the salt into its cation and anion components and then examine the possible reaction of each ion with water. (a) The cation is , which will hydrolyze to produce and . The anion is the conjugate base of the strong acid HI. Therefore. will not hydrolyze and the solution is acidic. (b) The cation does not hydrolyze. The is the conjugate base of the weak acid and will hydrolyze to give and . The solution will be basic. (c) is a small metal ion with a high charge and hydrolyzes to produce ions. The does not hydrolyze. Consequently, the solution will be acidic. (d) Both the and ions will hydrolyze. From Tables 16.5 and 16.3 we see that the of is greater than the for . Therefore, the solution will be acidic. Practice Exercise Predict whether the following solutions will be acidic, basic, or nearly neutral: (a) LiClO 4 , (b) Na , (c) , (d) .
16.14 Define . Write an equation relating and .
16.15 Calculate the hydrogen ion concentration for solutions with these values: (a) 2.42, (b) 11.21, (c) 6.96, (d) 15.00 . 16.16 Calculate the hydrogen ion concentration in moles per liter for each of these solutions: (a) a solution whose pH is 5.20 , (b) a solution whose is 16.00 , (c) a solution whose hydroxide concentration is .
Expert Answer
Acid is a substance which donates a proton.Base is a substance which accepts a proton.The acid base pair which differ by only one proton are called conjugate acid base pair.The acid contains one extra proton(H atom) and one more positive charge (or one fewer negative charge)compared to its conjugate base.similarly a conjugate base contains one fewer H atom and one more negative charge (or one fewer positive charge )compared to its conjugate acidCN- has one fewer H atom and one more negative charge than HCN. So,CN- is conjugate base of the acid HCN.HCN contains one extra proton(H atom) and one fewer negative charge compared toCN-.So,HCN is the conjugate acid of the base