(Solved): Ester Hydrolysis ester bond with a molecule of water, in this experiment an acidic hydrolysis of me ...
Ester Hydrolysis
ester bond with a molecule of water, in this experiment an acidic hydrolysis of methyl propionate was performed in order to form propanoic acid.
Propionic acid
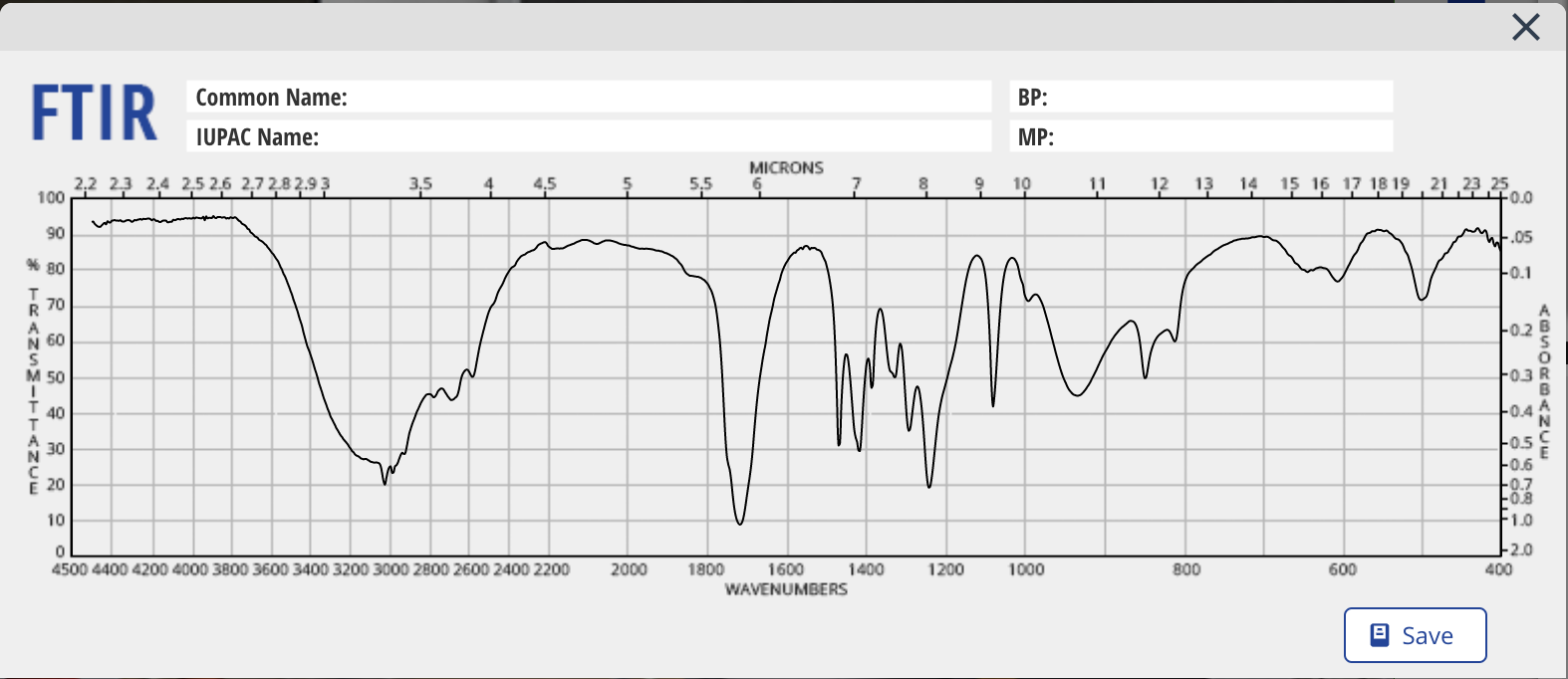
- IR SPECTRUM: numerate each absorption bands you consider relevant and draw the general structure of the functional group that band would correspond to (using R groups). For example, if there is an alcohol present, you can write "1. R-OH" under the band (legible!). That would mean that is it peak # 1 and that it was assigned to an alcohol.
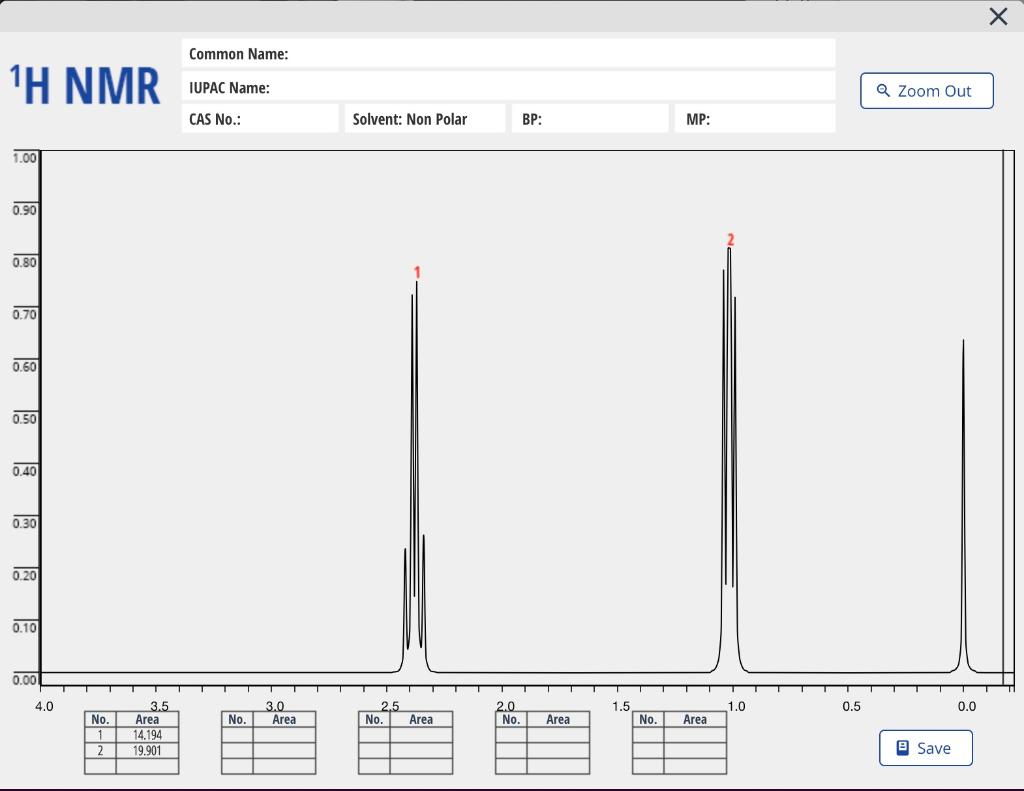
- H-NMR SPECTRUM. Draw the structure on the spectrum and assign each signal to a particular proton(s) in the molecule. Each signal has a number, so put that number next to the proton you think is giving that signal.
- write the name of the compound and draw its structure without any notations.
Please write on the spectrums and label all peaks and number them.
Expert Answer
solution :- . .starting materials : methyl propanoate, water and an acid as catalist (HCL) solvent used : dilute