Home /
Expert Answers /
Chemistry /
electrophilic-addition-of-mathrm-hbr-to-alkenes-yields-a-bromoalkane-the-reaction-begins-pa377
(Solved): Electrophilic addition of \( \mathrm{HBr} \) to alkenes yields a bromoalkane. The reaction begins ...
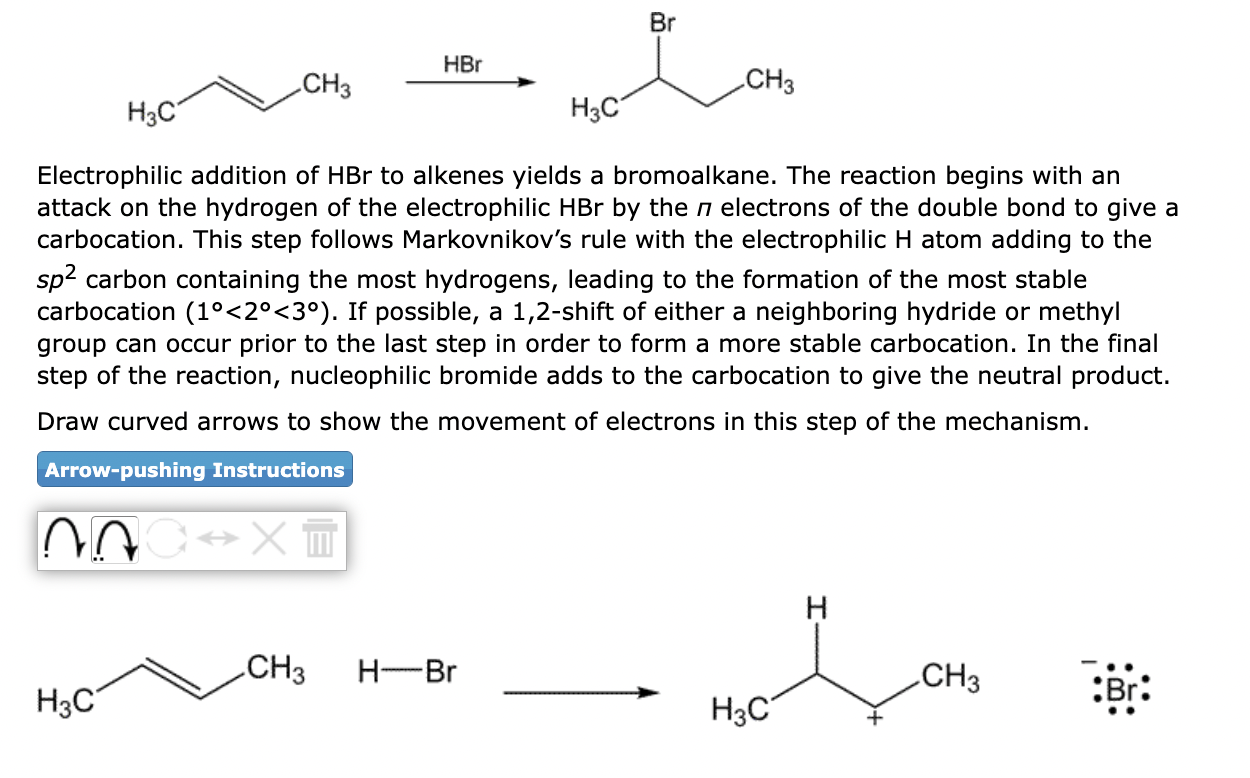
Electrophilic addition of \( \mathrm{HBr} \) to alkenes yields a bromoalkane. The reaction begins with an attack on the hydrogen of the electrophilic \( \mathrm{HBr} \) by the \( \pi \) electrons of the double bond to give a carbocation. This step follows Markovnikov's rule with the electrophilic \( \mathrm{H} \) atom adding to the \( s p^{2} \) carbon containing the most hydrogens, leading to the formation of the most stable carbocation \( \left(1^{\circ}<2^{\circ}<3^{\circ}\right) \). If possible, a 1,2 -shift of either a neighboring hydride or methyl group can occur prior to the last step in order to form a more stable carbocation. In the final step of the reaction, nucleophilic bromide adds to the carbocation to give the neutral product. Draw curved arrows to show the movement of electrons in this step of the mechanism.
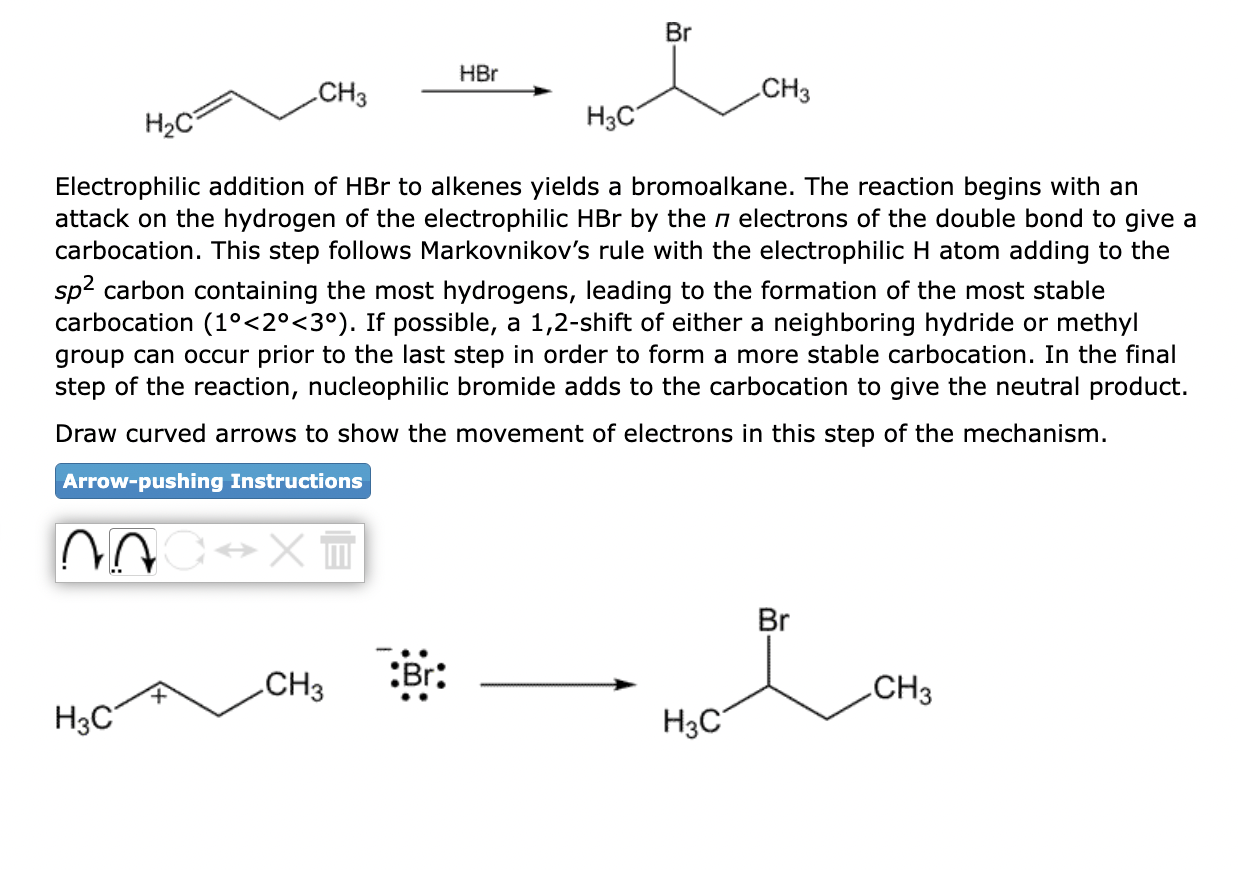
Electrophilic addition of \( \mathrm{HBr} \) to alkenes yields a bromoalkane. The reaction begins with an attack on the hydrogen of the electrophilic \( \mathrm{HBr} \) by the \( \pi \) electrons of the double bond to give a carbocation. This step follows Markovnikov's rule with the electrophilic \( \mathrm{H} \) atom adding to the \( s p^{2} \) carbon containing the most hydrogens, leading to the formation of the most stable carbocation \( \left(1^{\circ}<2^{\circ}<3^{\circ}\right) \). If possible, a 1,2 -shift of either a neighboring hydride or methyl group can occur prior to the last step in order to form a more stable carbocation. In the final step of the reaction, nucleophilic bromide adds to the carbocation to give the neutral product. Draw curved arrows to show the movement of electrons in this step of the mechanism.
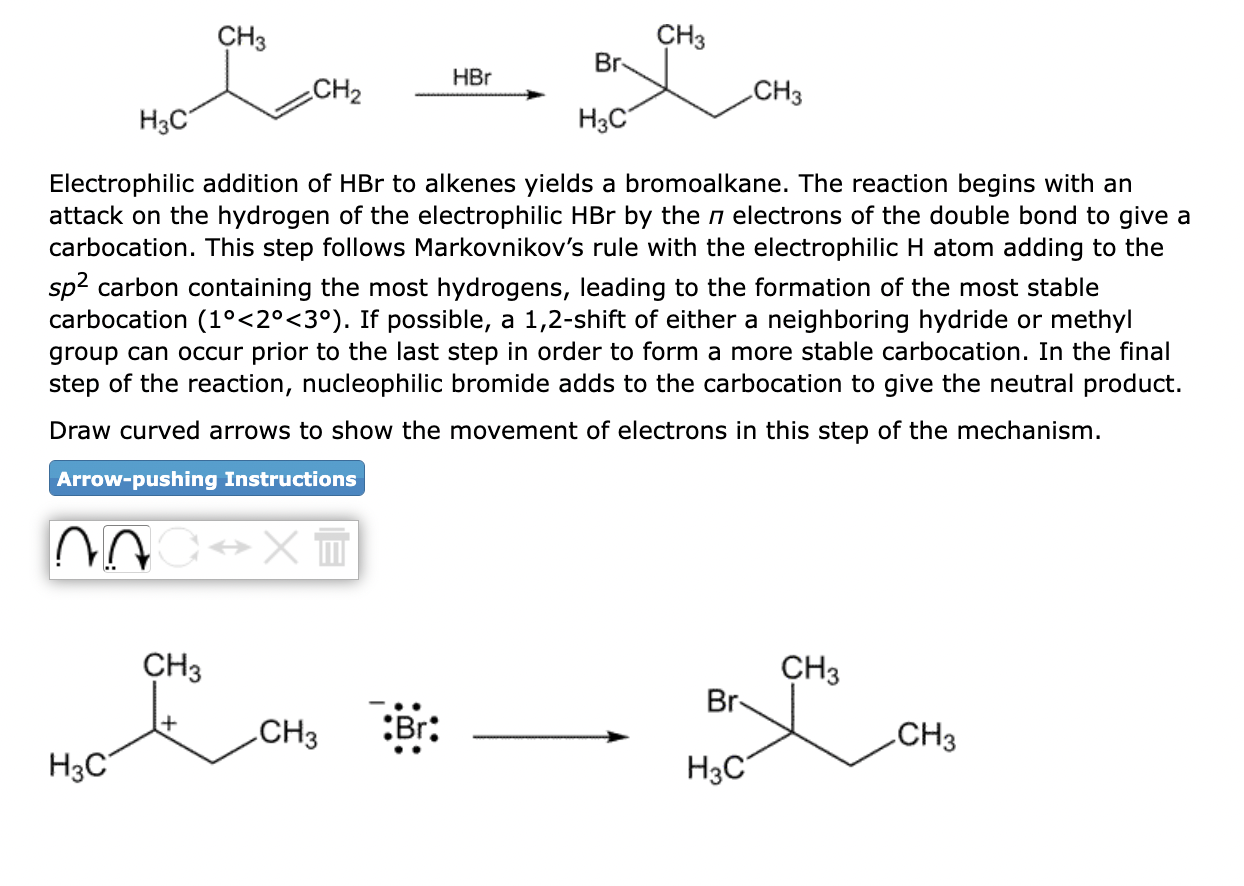
Electrophilic addition of \( \mathrm{HBr} \) to alkenes yields a bromoalkane. The reaction begins with an attack on the hydrogen of the electrophilic \( \mathrm{HBr} \) by the \( \pi \) electrons of the double bond to give a carbocation. This step follows Markovnikov's rule with the electrophilic \( \mathrm{H} \) atom adding to the \( s p^{2} \) carbon containing the most hydrogens, leading to the formation of the most stable carbocation \( \left(1^{\circ}<2^{\circ}<3^{\circ}\right) \). If possible, a 1,2 -shift of either a neighboring hydride or methyl group can occur prior to the last step in order to form a more stable carbocation. In the final step of the reaction, nucleophilic bromide adds to the carbocation to give the neutral product. Draw curved arrows to show the movement of electrons in this step of the mechanism.