Home /
Expert Answers /
Chemistry /
electrophilic-addition-of-hbr-to-alkenes-yields-a-bromoalkane-the-reaction-begins-with-an-attack-o-pa873
(Solved): Electrophilic addition of HBr to alkenes yields a bromoalkane. The reaction begins with an attack o ...
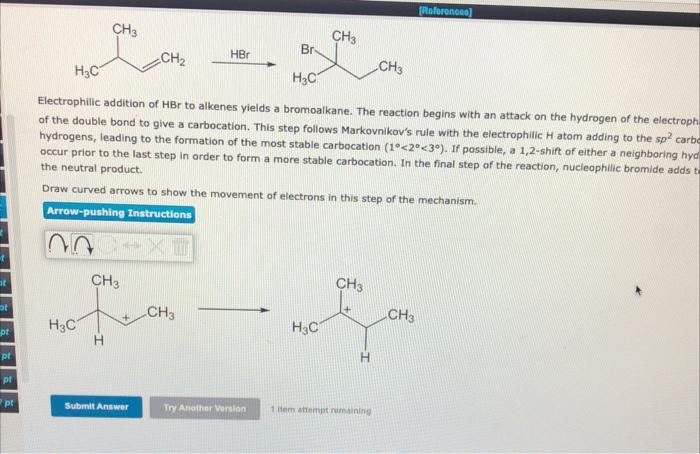

Electrophilic addition of HBr to alkenes yields a bromoalkane. The reaction begins with an attack on the hydrogen of the electroph of the double bond to give a carbocation. This step follows Markovnikov's rule with the electrophilic \( \mathrm{H} \) atom adding to the sp² carb hydrogens, leading to the formation of the most stable carbocation \( \left(1^{\circ}<2^{\circ}<3^{\circ}\right) \). If possible, a 1,2 -shift of either a neighboring hyc occur prior to the last step in order to form a more stable carbocation. In the final step of the reaction, nucleophilic bromide adds the neutral product. Draw curved arrows to show the movement of electrons in this step of the mechanism.
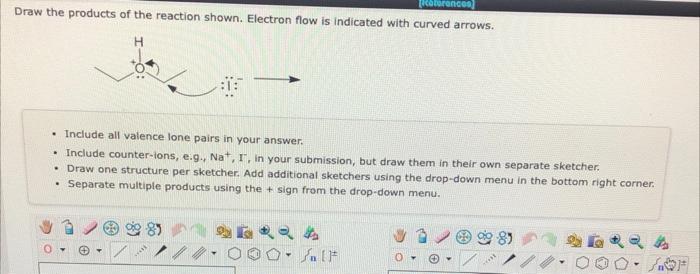
Draw the products of the reaction shown. Electron flow is indicated with curved arrows. - Include all valence lone pairs in your answer. - Include counter-ions, e.g.4 \( \mathrm{Na}^{+}, \mathrm{T} \), in your submission, but draw them in their own separate sketcher. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate multiple products using the \( + \) sign from the drop-down menu.
Draw the structures of the two carbocation intermediates that might form during the reaction of 2 -methyl-2-butene (above) with HBr. - You do hot have to consider stereochemistry. - Draw one structure per kketcher. Add additional sketchers using the drop-down menu in the bottom night corner. - Separate structures with is signs from the drop-down menu.