Home /
Expert Answers /
Chemistry /
each-copper-ii-sulfate-unit-is-associated-with-five-water-molecules-in-crystalline-copper-ii-sulf-pa260
(Solved): Each copper(II) sulfate unit is associated with five water molecules in crystalline copper(II) sulf ...
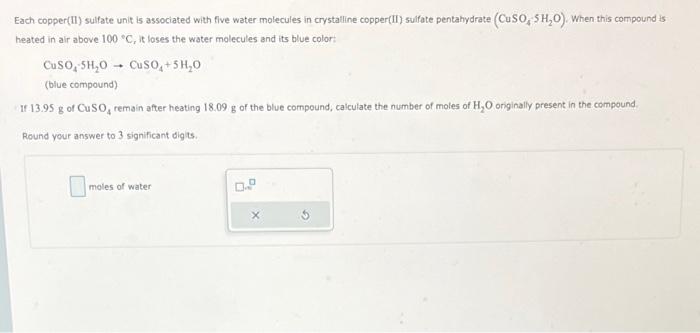
Each copper(II) sulfate unit is associated with five water molecules in crystalline copper(II) sulfate pentahydrate \( \left(\mathrm{CuSO}_{4}-5 \mathrm{H}_{2} \mathrm{O}\right) \). When this compound is heated in air above \( 100^{\circ} \mathrm{C} \), it loses the water molecules and its blue color: \[ \mathrm{CuSO}_{4}-5 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{CuSO}_{4}+5 \mathrm{H}_{2} \mathrm{O} \] (blue compound) If \( 13.95 \mathrm{~g} \) of \( \mathrm{CuSO}_{4} \) remain after heating \( 18.09 \mathrm{~g} \) of the blue compound, calculate the number of moles of \( \mathrm{H}_{2} \mathrm{O} \) originaly present in the compound. Round your answer to 3 significant digits. moles of water