Home /
Expert Answers /
Chemistry /
draw-three-dimensional-formulas-for-the-following-molecules-using-bold-and-dashed-wedge-bonds-where-pa333
(Solved): Draw three-dimensional formulas for the following molecules using bold and dashed wedge bonds where ...
Draw three-dimensional formulas for the following molecules using bold and dashed wedge bonds where appropriate. Indicate whether each bond in it is a \( \sigma \) or \( \pi \) bond, and provide the hybridization for each non-hydrogen atom. (a) \( \mathrm{CH}_{2} \mathrm{O} \) The carbon-oxygen double bond consists of \( \pi \) bond(s) and \( \sigma \) bond(s). The carbon orbitals are hybridized and the oxygen orbit no hybridized. two three four
\( \mathrm{H}_{2} \mathrm{C}=\mathrm{CHCH}=\mathrm{CH}_{2} \)
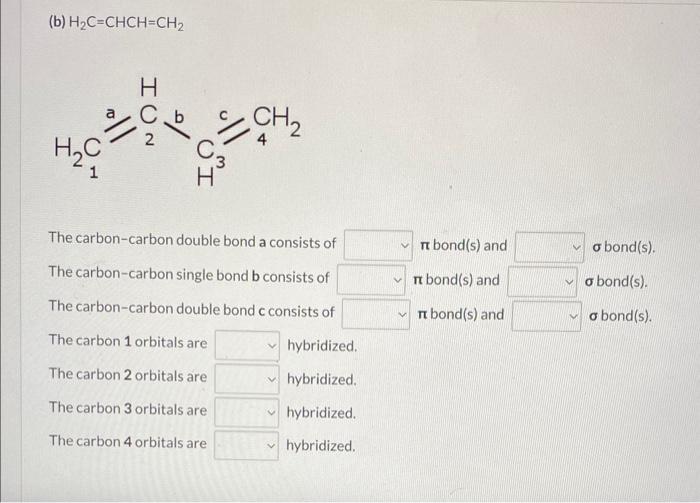
(b) \( \mathrm{H}_{2} \mathrm{C}=\mathrm{CHCH}=\mathrm{CH}_{2} \) \( \begin{array}{lll}\text { The carbon-carbon double bond a consists of } & \pi \text { bond(s) and } & \sigma \text { bond(s). } \\ \text { The carbon-carbon single bond b consists of } & \pi \text { bond(s) and } & \sigma \text { bond(s). } \\ \text { The carbon-carbon double bond c consists of } & \pi \text { bond(s) and } & \sigma \text { bond(s). }\end{array} \) The carbon 1 orbitals are hybridized. The carbon 2 orbitals are hybridized. The carbon 3 orbitals are hybridized. The carbon 4 orbitals are hybridized.
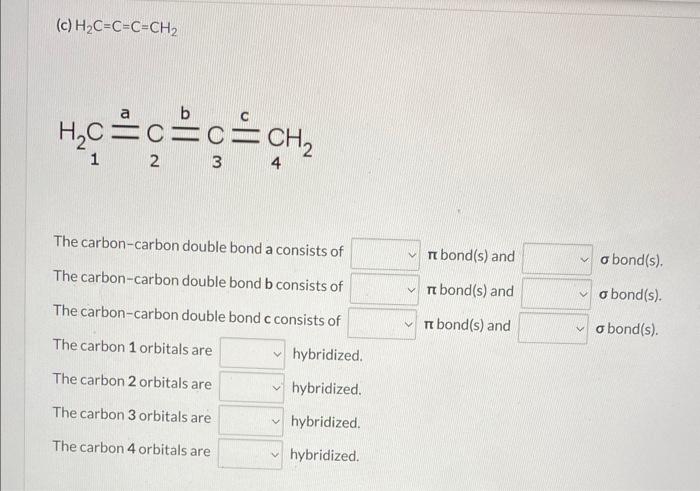
\( \mathrm{H}_{2} \mathrm{C}=\mathrm{C}=\mathrm{C}=\mathrm{CH}_{2} \)
(c) \( \mathrm{H}_{2} \mathrm{C}=\mathrm{C}=\mathrm{C}=\mathrm{CH}_{2} \) \[ \mathrm{H}_{2} \mathrm{C} \underset{2}{\mathrm{C}} \underset{2}{\mathrm{C}}=\underset{4}{\mathrm{C}} \underset{3}{=} \mathrm{CH}_{2} \] \begin{tabular}{l|l|l} The carbon-carbon double bond a consists of & \( \pi \) bond(s) and & \( \boldsymbol{\sigma} \) bond(s). \\ The carbon-carbon double bond b consists of & \( \pi \) bond(s) and & \( \boldsymbol{\sigma} \) bond(s). \\ The carbon-carbon double bond c consists of & \( \pi \) bond(s) and & \( \boldsymbol{\sigma} \) bond(s). \end{tabular} The carbon 1 orbitals are hybridized. The carbon 2 orbitals are hybridized. The carbon 3 orbitals are hybridized. The carbon 4 orbitals are hybridized.
Expert Answer
Solution of part a is in this step as b