Home /
Expert Answers /
Chemistry /
draw-the-organic-molecule-s-which-is-are-formed-in-the-following-reaction-pa610
(Solved): Draw the organic molecule(s) which is(are) formed in the following reaction. ...
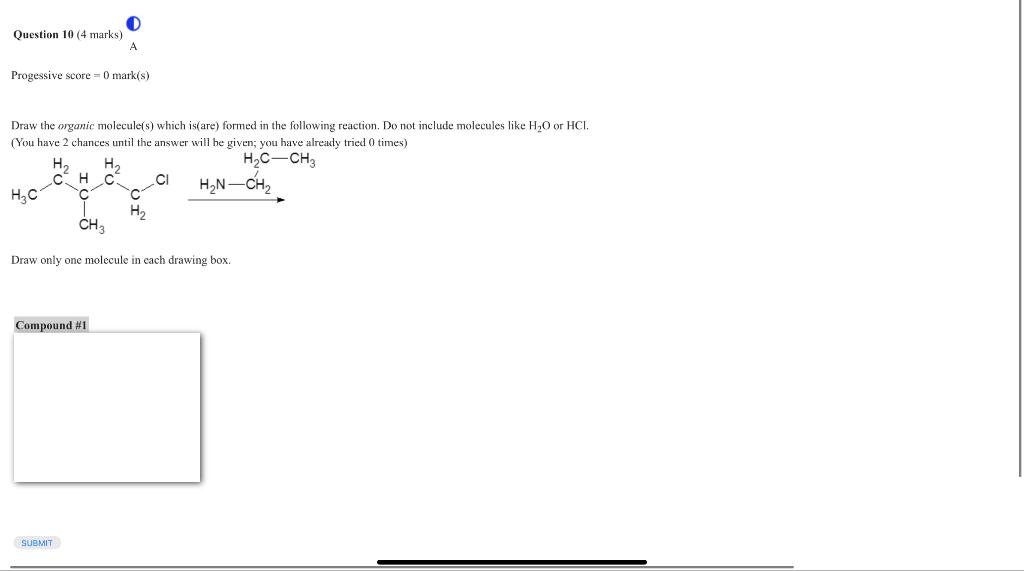
Draw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like or . (You have 2 chances until the answer will be given; you have already tried 0 times) Draw only one molecule in each drawing box.
Draw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like or . (You have 2 chances until the answer will be given; you have already tried 0 times) Draw only one molecule in each drawing box.
Draw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like or . (You have 2 chances until the answer will be given; you have already tried 0 times) Draw only one molecule in each drawing box.
A. (Worth: 2 marks) The following is a nucleophilic substitution reaction of R-3-bromo-2-methylhexane with . The obtical rotation of the solution after the reaction was complete was Select which of the following statements is correct: The mechanism for this reaction is because the product is a racemic mixture of enantiomers because the solution optical rotation was because the carbon attached to the leaving group, the bromine atom, is a primary carbon (a carbon with one carbons attached to it because the nucleophile, reacts via this mechanism due to its size because the steric crowding around the carbon attached to the leaving group, the bromine atom, is a minimum.
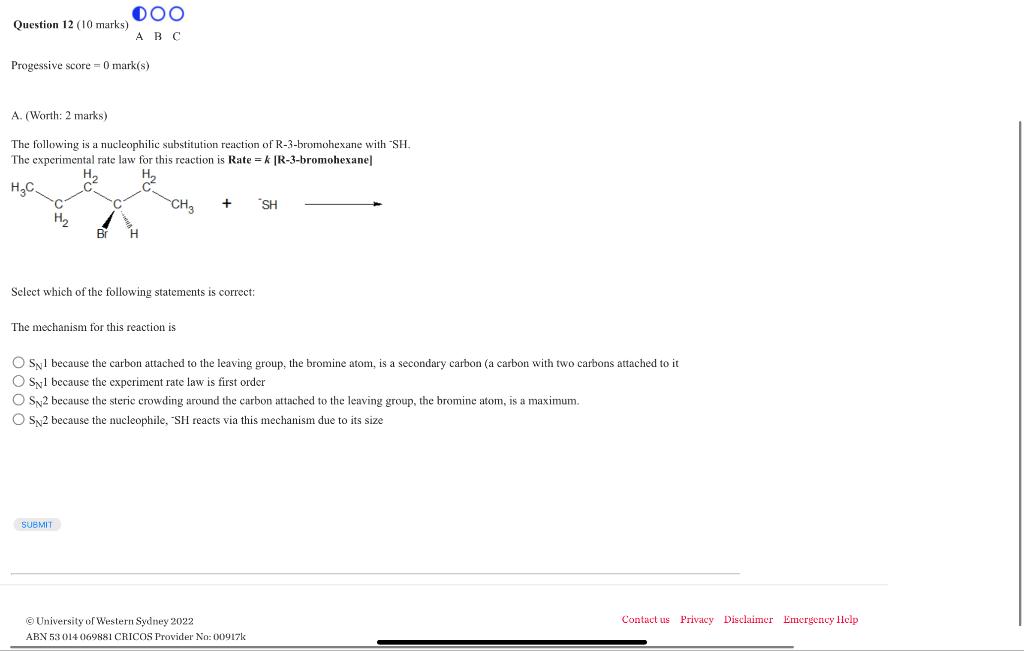
The following is a nucleophilic substitution reaction of -bromohexane with . The experimental rate law for this reaction is [R-3-bromohexane] Select which of the following statements is correct: The mechanism for this reaction is because the carbon attached to the leaving group, the bromine atom, is a secondary carbon (a carbon with two carbons attached to it because the experiment rate law is first order because the steric crowding around the carbon attached to the leaving group, the bromine atom, is a maximum. because the nucleophile, reacts via this mechanism due to its size (c) University of Western Sydney 2022 Contact us Priv ABN 53014069881 CRICOS Provider No:
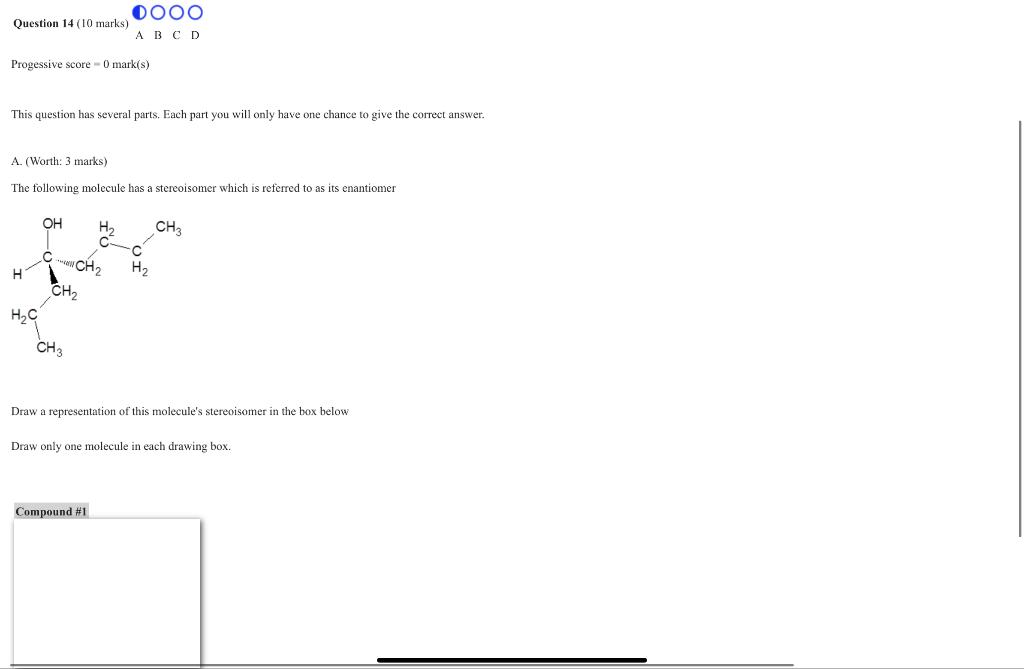
This question has several parts. Each part you will only have one chance to give the correct answer. A. (Worth: 3 marks) The following molecule has a stereoisomer which is referred to as its enantiomer Draw a representation of this molecule's stereoisomer in the box below Draw only one molecule in each drawing box.
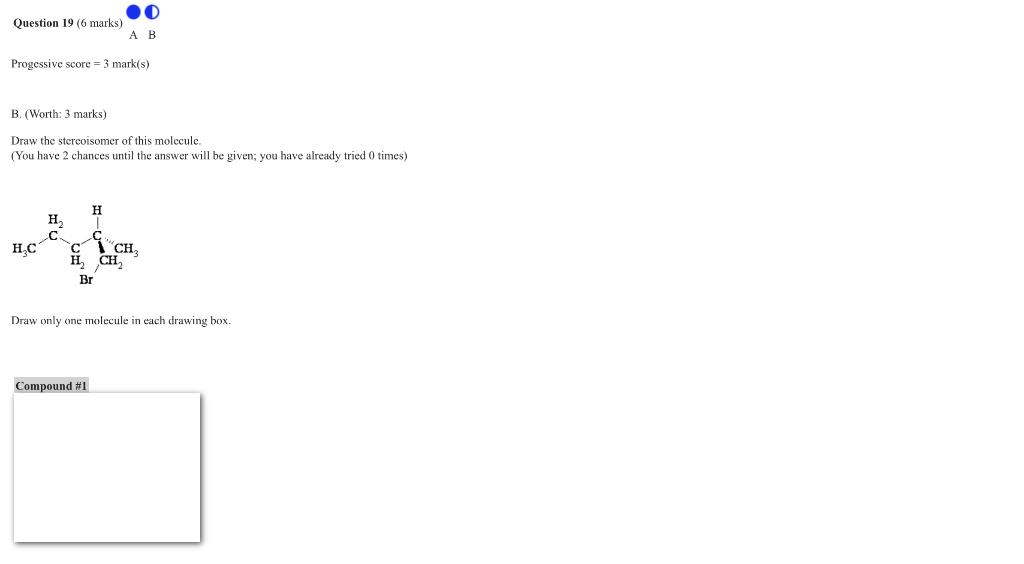
B. (Worth: 3 marks) Draw the stereoisomer of this molecule. (You have 2 chances until the answer will be given; you have already tried 0 times) Draw only one molecule in each drawing box.
B. (Worth: 3 marks) Draw the stereoisomer of this molecule. (You have 2 chances until the answer will be given; you have already tried 0 times) Draw only one molecule in each drawing box.
Draw the molecule in the box below that has the IUPAC name, R-4-ethyl-2-methyloctane (You have three chances until the answer will be given; you have already tried 0 times) Draw only one molecule in each drawing box.