Home /
Expert Answers /
Chemistry /
draw-the-lewis-structures-for-three-resonance-forms-of-the-nitrate-ion-no3-include-electron-pa558
(Solved): Draw the Lewis structures for three resonance forms of the nitrate ion, NO3. Include electron ...
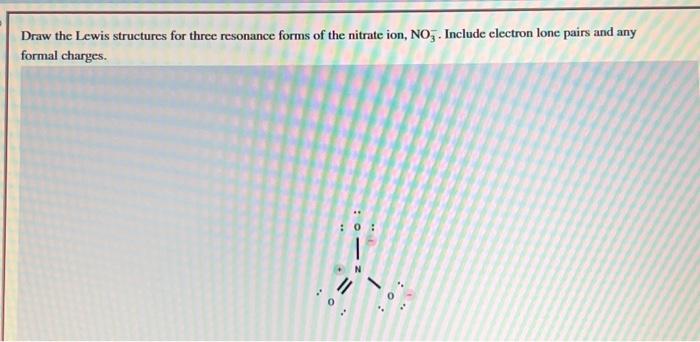
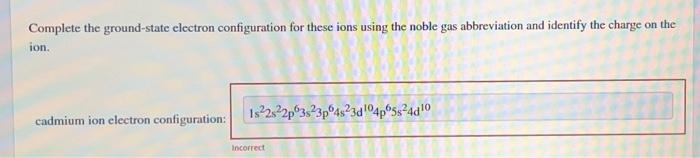
Draw the Lewis structures for three resonance forms of the nitrate ion, . Include electron lone pairs and any formal charges.
Complete the ground-state electron configuration for these ions using the noble gas abbreviation and identify the charge on the ion. cadmium ion electron configuration:
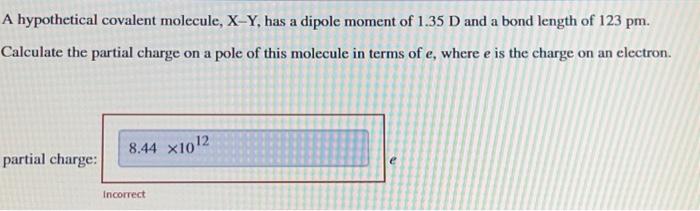
A hypothetical covalent molecule, , has a dipole moment of and a bond length of . Calculate the partial charge on a pole of this molecule in terms of , where is the charge on an electron.