Home /
Expert Answers /
Chemistry /
draw-the-lewis-structure-for-nitryl-fluoride-chemical-formula-nof-determine-if-the-following-pa343
(Solved): Draw the Lewis structure for nitryl fluoride (chemical formula; NOF). Determine if the following ...
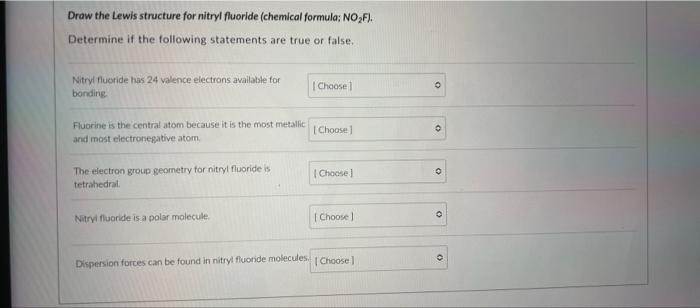
Draw the Lewis structure for nitryl fluoride (chemical formula; NO?F). Determine if the following statements are true or false. Nitryl fluoride has 24 valence electrons available for bonding Fluorine is the central atom because it is the most metallic and most electronegative atom The electron group geometry for nitryl fluoride is tetrahedral. Nitryl fluoride is a polar molecule. [Choose] [Choose] [Choose] [Choose] Dispersion forces can be found in nitryl fluoride molecules [ Choose ] 0 0 O 0
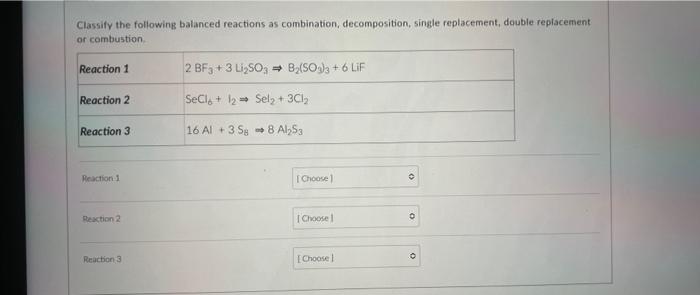
Classify the following balanced reactions as combination, decomposition, single replacement, double replacement or combustion. Reaction 1 Reaction 2 Reaction 3 Reaction 1 Reaction 2 Reaction 3 2 BF3+ 3 Li?SO3 ? B?(SO3)3 +6 LiF SeCl + 12 Sel? + 3Cl? 16 Al +3 Sg 8 Al?53 [Choose] [Choose [Choose 0 10
Expert Answer
Answer: Lewis structure of Nitr