Home /
Expert Answers /
Chemistry /
draw-the-lewis-structure-for-bf3-including-lone-pairs-what-is-the-fbf-bond-angle-109-5-pa139
(Solved): Draw the Lewis structure for BF3, including lone pairs. What is the FBF bond angle? 109.5 ...
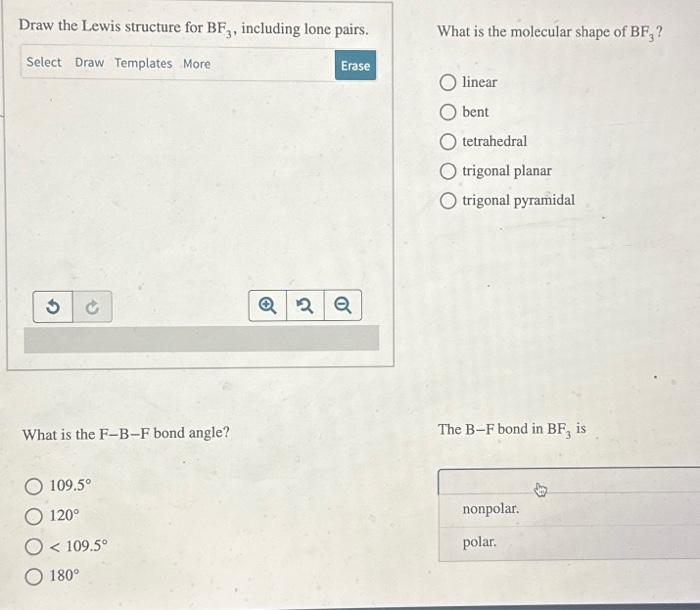

Draw the Lewis structure for , including lone pairs. What is the bond angle? What is the molecular shape of ? linear bent tetrahedral trigonal planar trigonal pyramidal The bond in is nonpolar. polar.
The molecule is nonpolar. polar.