Home /
Expert Answers /
Chemistry /
draw-lewis-structure-s-showing-all-possible-equivalent-resonance-forms-for-the-carbonate-ion-pa715
(Solved): Draw Lewis structure(s) showing all possible equivalent resonance forms for the carbonate ion ( \( ...
![atom to obey the octet rule.
\[
\mathrm{CH}_{3} \mathrm{COO}^{-}:
\]](https://media.cheggcdn.com/study/b10/b1018e3d-7fa9-4ae0-9ddf-eaee7537518e/image)
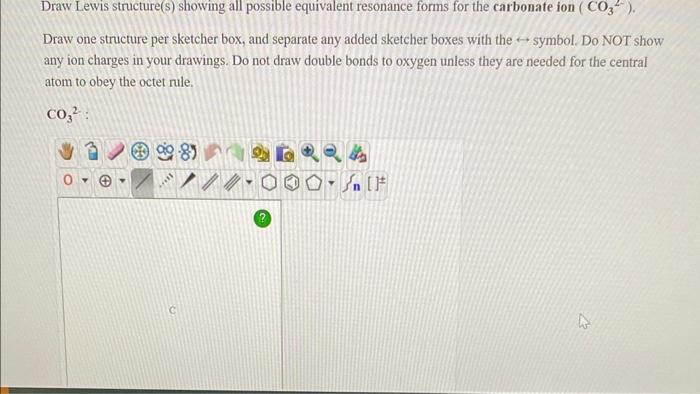
Draw Lewis structure(s) showing all possible equivalent resonance forms for the carbonate ion ( \( \mathrm{CO}_{3}{ }^{2-} \) ). Draw one structure per sketcher box, and separate any added sketcher boxes with the \( \leftrightarrow \) symbol. Do NOT show any ion charges in your drawings. Do not draw double bonds to oxygen unless they are needed for the central atom to obey the octet rule.
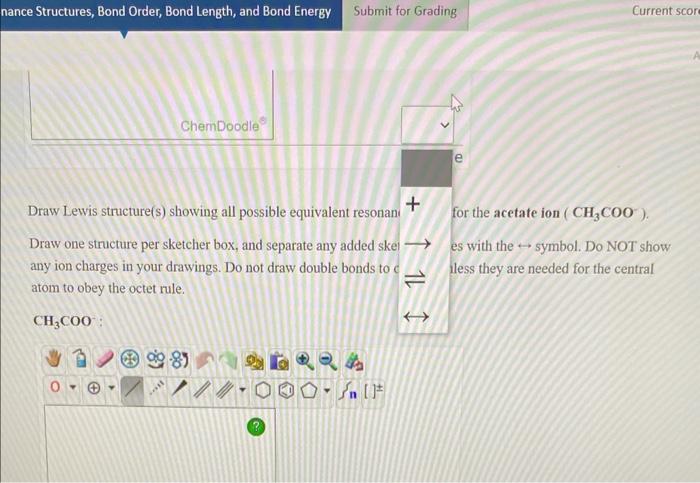
Draw Lewis structure(s) showing all possible equivalent resonani \( + \) for the acetate ion \( \left(\mathrm{CH}_{3} \mathrm{COO}^{-}\right) \). Draw one structure per sketcher box, and separate any added skel \( \longrightarrow \) es with the \( \hookrightarrow \) symbol. Do NOT show any ion charges in your drawings. Do not draw double bonds to \( \mathrm{C} \rightleftharpoons \) less they are needed for the central atom to obey the octet rule.
atom to obey the octet rule. \[ \mathrm{CH}_{3} \mathrm{COO}^{-}: \]
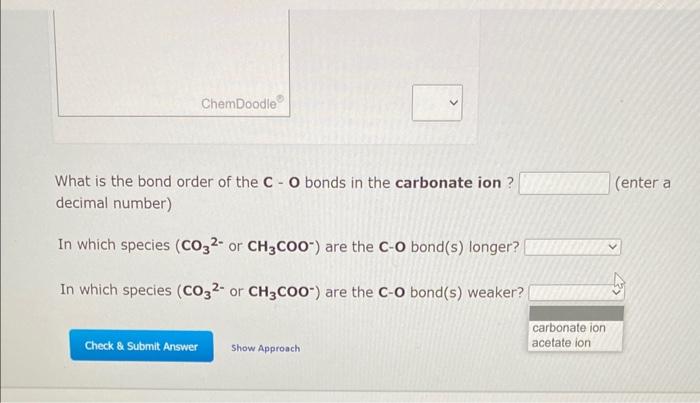
What is the bond order of the \( \mathbf{C} \) - \( \mathbf{O} \) bonds in the carbonate ion? (enter a decimal number) In which species \( \left(\mathrm{CO}_{3}^{2-}\right. \) or \( \left.\mathbf{C H}_{3} \mathrm{COO}^{-}\right) \)are the \( \mathbf{C} \) - \( \mathbf{O} \) bond(s) longer? In which species \( \left(\mathrm{CO}_{3}^{2-}\right. \) or \( \left.\mathbf{C H}_{3} \mathbf{C O O}^{-}\right) \)are the \( \mathbf{C}-\mathbf{O} \) bond(s) weaker?