Home /
Expert Answers /
Chemistry /
draw-a-galvanic-cell-in-this-activity-you-will-draw-and-label-diagrams-of-galvanic-cells-the-firs-pa236
(Solved): Draw a Galvanic Cell In this activity, you will draw and label diagrams of galvanic cells. The firs ...
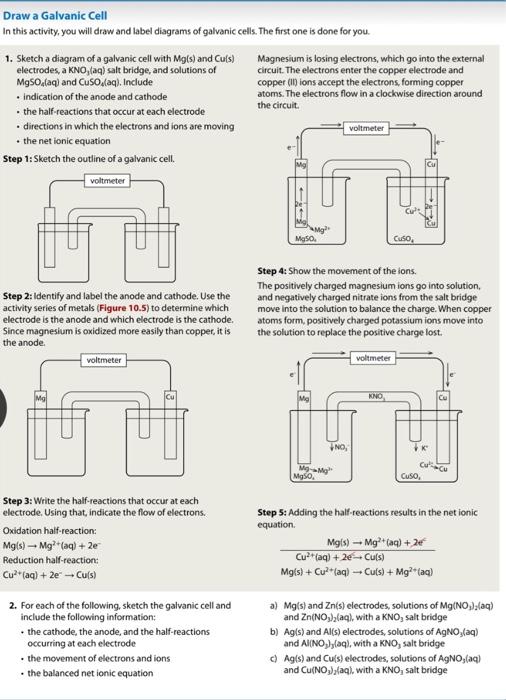
Draw a Galvanic Cell In this activity, you will draw and label diagrams of galvanic cells. The first one is done for you. 1. Sketch a diagram of a galvanic cell with Mg(s) and Cu(s) electrodes, a KNO, (aq) salt bridge, and solutions of MgSO4(aq) and CuSO,(aq). Include • indication of the anode and cathode • the half-reactions that occur at each electrode • directions in which the electrons and ions are moving • the net ionic equation Step 1: Sketch the outline of a galvanic cell. voltmeter Step 2: Identify and label the anode and cathode. Use the activity series of metals (Figure 10.5) to determine which electrode is the anode and which electrode is the cathode. Since magnesium is oxidized more easily than copper, it is the anode. voltmeter Cu Step 3: Write the half-reactions that occur at each electrode. Using that, indicate the flow of electrons. Oxidation half-reaction: Mg(s) ? Mg²+ (aq) + 2e Reduction half-reaction: Cu²+ (aq) + 2e ?? Cu(s) 2. For each of the following, sketch the galvanic cell and include the following information: . the cathode, the anode, and the half-reactions occurring at each electrode • the movement of electrons and ions • the balanced net ionic equation Magnesium is losing electrons, which go into the external circuit. The electrons enter the copper electrode and copper (II) ions accept the electrons, forming copper atoms. The electrons flow in a clockwise direction around the circuit. voltmeter Mg Mg50. CuO, Step 4: Show the movement of the ions. The positively charged magnesium ions go into solution, and negatively charged nitrate ions from the salt bridge move into the solution to balance the charge. When copper atoms form, positively charged potassium ions move into the solution to replace the positive charge lost. voltmeter KNO Mg K Cu-Cu Mg Mg Mg50, Cuso, Step 5: Adding the half-reactions results in the net ionic equation. Mg(s) Mg²+ (aq) + 2€ Cu²+ (aq) + 2e ? Cu(s) Mg(s) + Cu²+ (aq) ? Cu(s) + Mg²+ (aq) a) Mg(s) and Zn(s) electrodes, solutions of Mg(NO?),(aq) and Zn(NO?)?(aq), with a KNO, salt bridge b) Ag(s) and Al(s) electrodes, solutions of AgNO,(aq) and Al(NO?),(aq), with a KNO, salt bridge c) Ag(s) and Cu(s) electrodes, solutions of AgNO,(aq) and Cu(NO?),(aq), with a KNO, salt bridge NO,