Home /
Expert Answers /
Chemistry /
drag-conversion-units-onto-the-boxes-in-the-equation-to-make-conversions-some-boxes-can-be-left-e-pa624
(Solved): Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left e ...
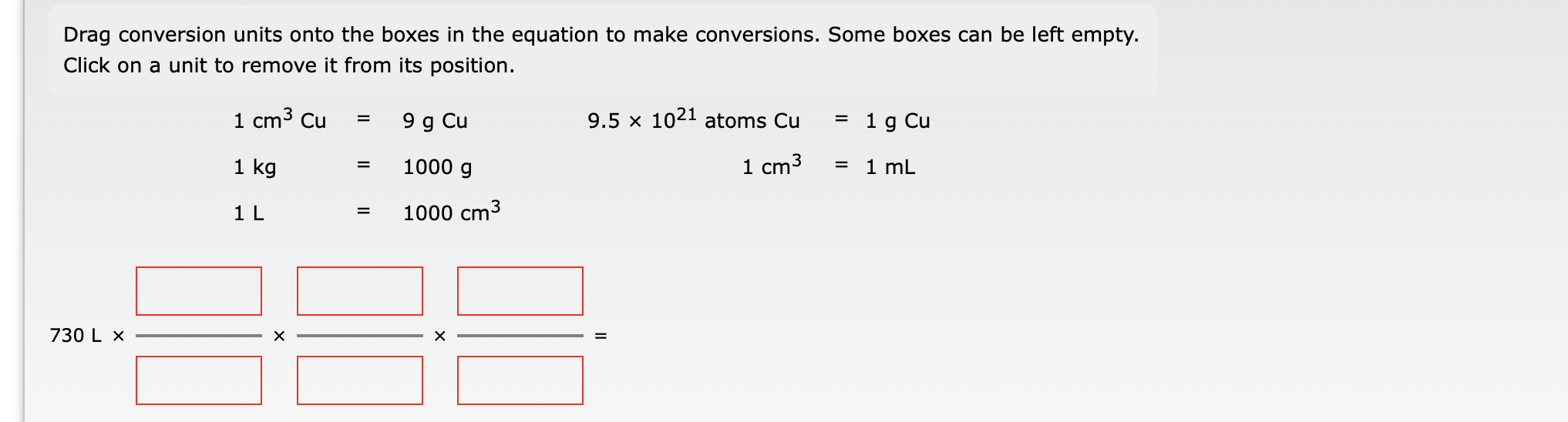
Drag conversion units onto the boxes in the equation to make conversions. Some boxes can be left empty. Click on a unit to remove it from its position. \( \begin{array}{rlrl}1 \mathrm{~cm}^{3} \mathrm{Cu} & =9 \mathrm{~g} \mathrm{Cu} & 9.5 \times 10^{21} \text { atoms } \mathrm{Cu} & =1 \mathrm{~g} \\ 1 \mathrm{~kg} & =1000 \mathrm{~g} & 1 \mathrm{~cm}^{3}=1 \mathrm{~m} \\ 1 \mathrm{~L} & =1000 \mathrm{~cm}^{3} & \end{array} \) \[ 730 \mathrm{~L} \times=\times 1 \times= \]