Home /
Expert Answers /
Chemistry /
does-a-reaction-occur-when-aqueous-solutions-of-iron-iii-bromide-and-silver-i-nitrate-are-combin-pa936
(Solved): Does a reaction occur when aqueous solutions of iron(III) bromide and silver(I) nitrate are combin ...



Does a reaction occur when aqueous solutions of iron(III) bromide and silver(I) nitrate are combined? Oyes no If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
Does a reaction occur when aqueous solutions of lead (II) acetate and copper (II) chloride are combined? Oyes no If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.
Peaction occur when aqueous solutions of potassium nitrate and copper(II) chloride are combined? Oyes no If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. -
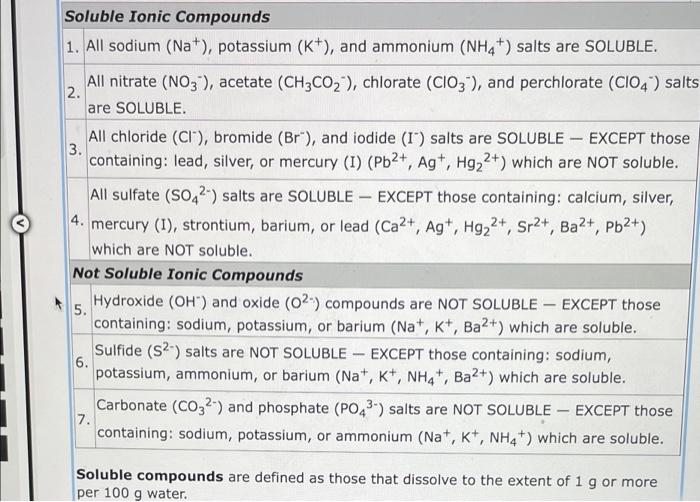
Soluble Ionic Compounds 1. All sodium (Na+), potassium (K+), and ammonium (NH4+) salts are SOLUBLE. 2. All nitrate (NO3), acetate (CH3CO?), chlorate (CIO3), and perchlorate (CIO4) salts are SOLUBLE. - 3. All chloride (CI), bromide (Br"), and iodide (I) salts are SOLUBLE EXCEPT those containing: lead, silver, or mercury (I) (Pb2+, Ag+, Hg?2+) which are NOT soluble. All sulfate (SO42) salts are SOLUBLE EXCEPT those containing: calcium, silver, 4. mercury (1), strontium, barium, or lead (Ca2+, Ag+, Hg?2+, Sr2+, Ba²+, Pb²+) which are NOT soluble. Not Soluble Ionic Compounds 5. Hydroxide (OH) and oxide (02) compounds are NOT SOLUBLE - EXCEPT those containing: sodium, potassium, or barium (Na+, K+, Ba2+) which are soluble. Sulfide (S2) salts are NOT SOLUBLE- EXCEPT those containing: sodium, potassium, ammonium, or barium (Na+, K+, NH4+, Ba2+) which are soluble. - 6. 7. Carbonate (CO32-) and phosphate (PO4³-) salts are NOT SOLUBLE EXCEPT those containing: sodium, potassium, or ammonium (Na+, K+, NH4+) which are soluble. Soluble compounds are defined as those that dissolve to the extent of 1 g or more per 100 g water.