Home /
Expert Answers /
Chemistry /
dna-concentration-the-nucleotide-bases-of-dna-and-rna-are-aromatic-and-absorb-ultraviolet-light-pa676
(Solved): DNA concentration. The nucleotide bases of DNA and RNA are aromatic and absorb ultraviolet light. ...
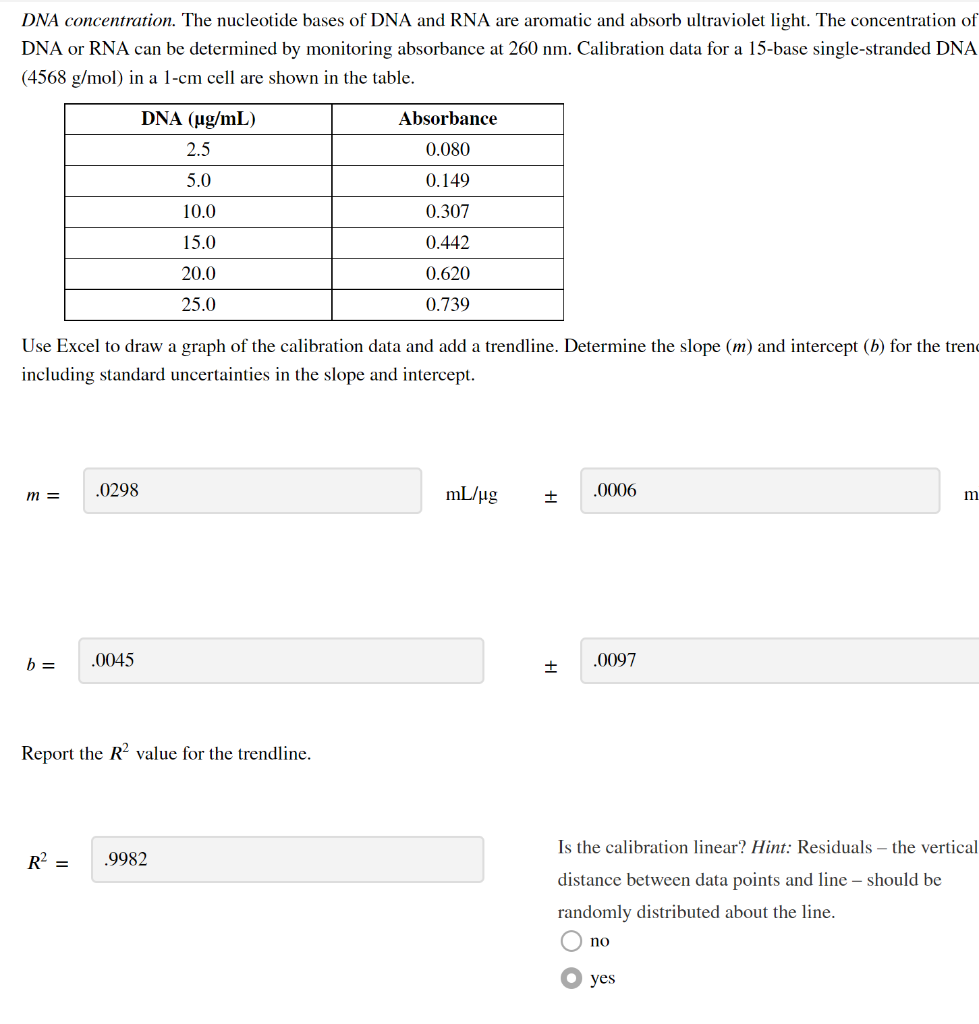
DNA concentration. The nucleotide bases of DNA and RNA are aromatic and absorb ultraviolet light. The concentration of DNA or RNA can be determined by monitoring absorbance at \( 260 \mathrm{~nm} \). Calibration data for a 15-base single-stranded DNA \( (4568 \mathrm{~g} / \mathrm{mol}) \) in a \( 1-\mathrm{cm} \) cell are shown in the table. Use Excel to draw a graph of the calibration data and add a trendline. Determine the slope \( (m) \) and intercept \( (b) \) for the tren including standard uncertainties in the slope and intercept. \[ m=\quad \mathrm{mL} / \mu \mathrm{g} \quad \pm \] \[ b=\quad \pm \] Report the \( R^{2} \) value for the trendline. \[ R^{2}= \] Is the calibration linear? Hint: Residuals - the vertical distance between data points and line - should be randomly distributed about the line. no yes
What is the molar absorptivity of the single-stranded DNA? Incorrect The absorptivity of DNA and RNA depends on the nucleotides present and their sequence. \( { }^{13} \) A popular online encyclopedia says absorptivity for all single-stranded DNA is \( 0.027(\mu \mathrm{g} / \mathrm{mL})^{-1} \mathrm{~cm}^{-1} \). How much error would result if the web site value were used for this DNA sample? error: Incorrect Analysis of a DNA sample in triplicate with a hand-held photometer yielded a mean concentration of \( 8.254 \mu \mathrm{g} / \mathrm{mL} \) and standard deviation of \( 0.019 \mu \mathrm{g} / \mathrm{mL} \). A commercial spectrometer gave \( 8.373 \pm 0.139 \mu \mathrm{g} / \mathrm{mL} \) for \( n=3 \). Use the \( F \)-test to determine whether the precisions of the hand-held photometer and the commercial spectrometer are statistically equivalent at the \( 95 \% \) confidence level. Report the calculated \( F \) value and the \( F \) value from the table. Are the precisions statistically equivalent at the \( 95 \% \) confidence level? no yes
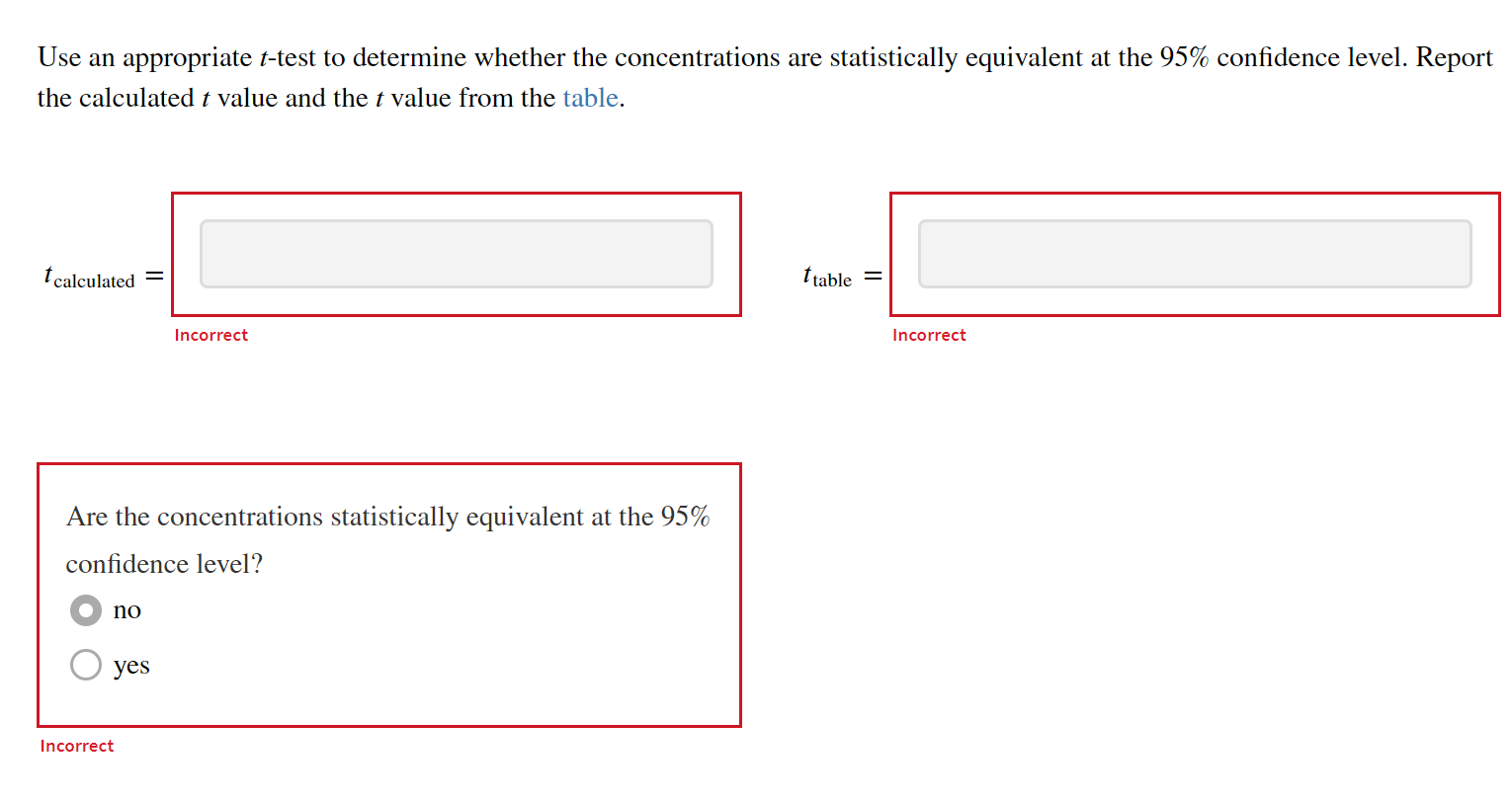
Use an appropriate \( t \)-test to determine whether the concentrations are statistically equivalent at the \( 95 \% \) confidence level. Report the calculated \( t \) value and the \( t \) value from the table. \[ t_{\text {table }}= \] Incorrect Incorrect Are the concentrations statistically equivalent at the \( 95 \% \) confidence level? no yes
Expert Answer
As you asked for the conversion of the units all