Home /
Expert Answers /
Chemistry /
diffusion-partial-pressure-mole-fraction-three-glass-bulbs-joined-by-closed-stopcocks-have-pa502
(Solved): Diffusion -- Partial Pressure -- Mole Fraction Three glass bulbs, joined by closed stopcocks, have ...
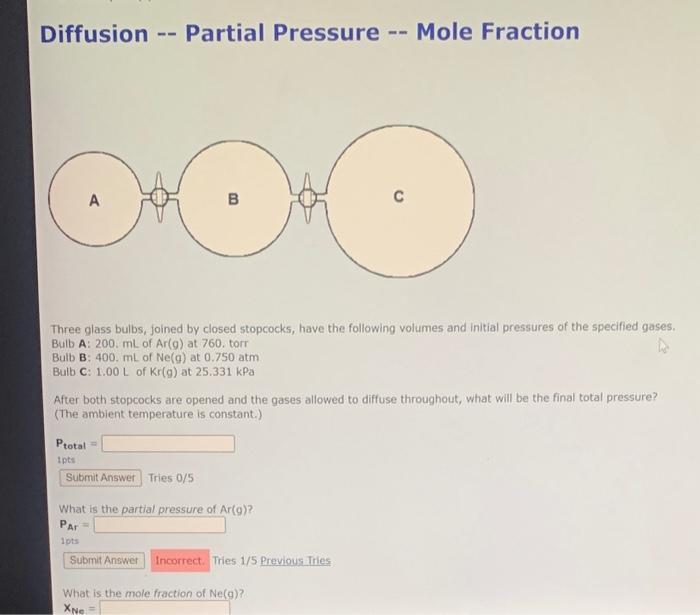
Diffusion -- Partial Pressure -- Mole Fraction Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases. Bulb A: 200. mL of Ar(g) at 760. tor Bulb B: \( 400 . \mathrm{mL} \) of \( \mathrm{Ne}(\mathrm{g}) \) at \( 0.750 \mathrm{~atm} \) Bulb C: \( 1.00 \mathrm{~L} \) of \( \mathrm{Kr}(\mathrm{g}) \) at \( 25.331 \mathrm{kPa} \) After both stopcocks are opened and the gases allowed to diffuse throughout, what will be the final total pressure? (The ambient temperature is constant.) \[ P_{\text {total }}= \] ipts Tries \( 0 / 5 \) What is the partial pressure of \( \operatorname{Ar}(9) ? \) \( \mathrm{P}_{\mathrm{Ar}}= \) 1pts Tries 1/5 Previous Tries What is the mole fraction of Ne(a)? \( x_{\mathrm{Ne}}= \)