Home /
Expert Answers /
Chemistry /
determine-the-rate-law-and-the-value-of-k-for-the-following-reaction-using-the-data-provided-2n-2-pa678
(Solved): Determine the rate law and the value of k for the following reaction using the data provided. 2N_(2) ...
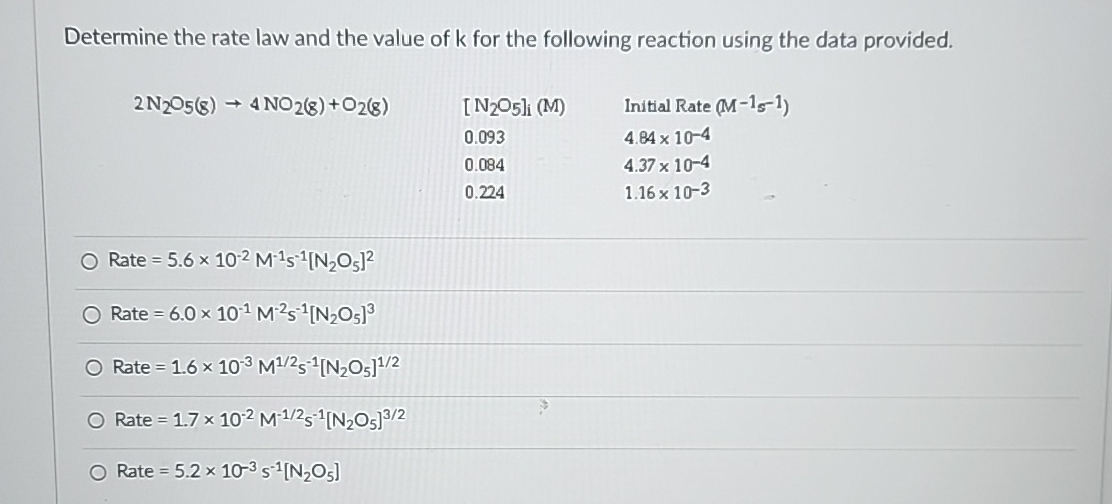
Determine the rate law and the value of
kfor the following reaction using the data provided.
2N_(2)O_(5)(g)->4NO_(2)(g)+O_(2)(g)]
(M)
Initial Rate (M^(-1)s^(-1))
4.84\times 10^(-4)
4.37\times 10^(-4)
1.16\times 10^(-3)Rate
=5.6\times 10^(-2)M^(-1)s^(-1)[N_(2)O_(5)]^(2)Rate
=6.0\times 10^(-1)M^(-2)s^(-1)[N_(2)O_(5)]^(3)Rate
=1.6\times 10^(-3)M^((1)/(2))S^(-1)[N_(2)O_(5)]^((1)/(2))Rate
=1.7\times 10^(-2)M^(-(1)/(2))s^(-1)[N_(2)O_(5)]^((3)/(2))Rate
=5.2\times 10^(-3)s^(-1)[N_(2)O_(5)]