Home /
Expert Answers /
Chemistry /
determine-the-pka-s-and-the-ka-s-of-the-acid-you-worked-with-this-will-require-you-to-determine-pa788
(Solved): Determine the pKa(s) and the Ka(s) of the acid you worked with. This will require you to determine ...
Determine the pKa(s) and the Ka(s) of the acid you worked with.
This will require you to determine your titration's equivalence and half-equivalence point(s), including the solution pH these were reached at.
(please show work, I'm very stuck on this)
Data:
NaOH concentration: 0.3M
Maliec acid concentration: 0.25M
(total of 10mL together)
50mL of DI water
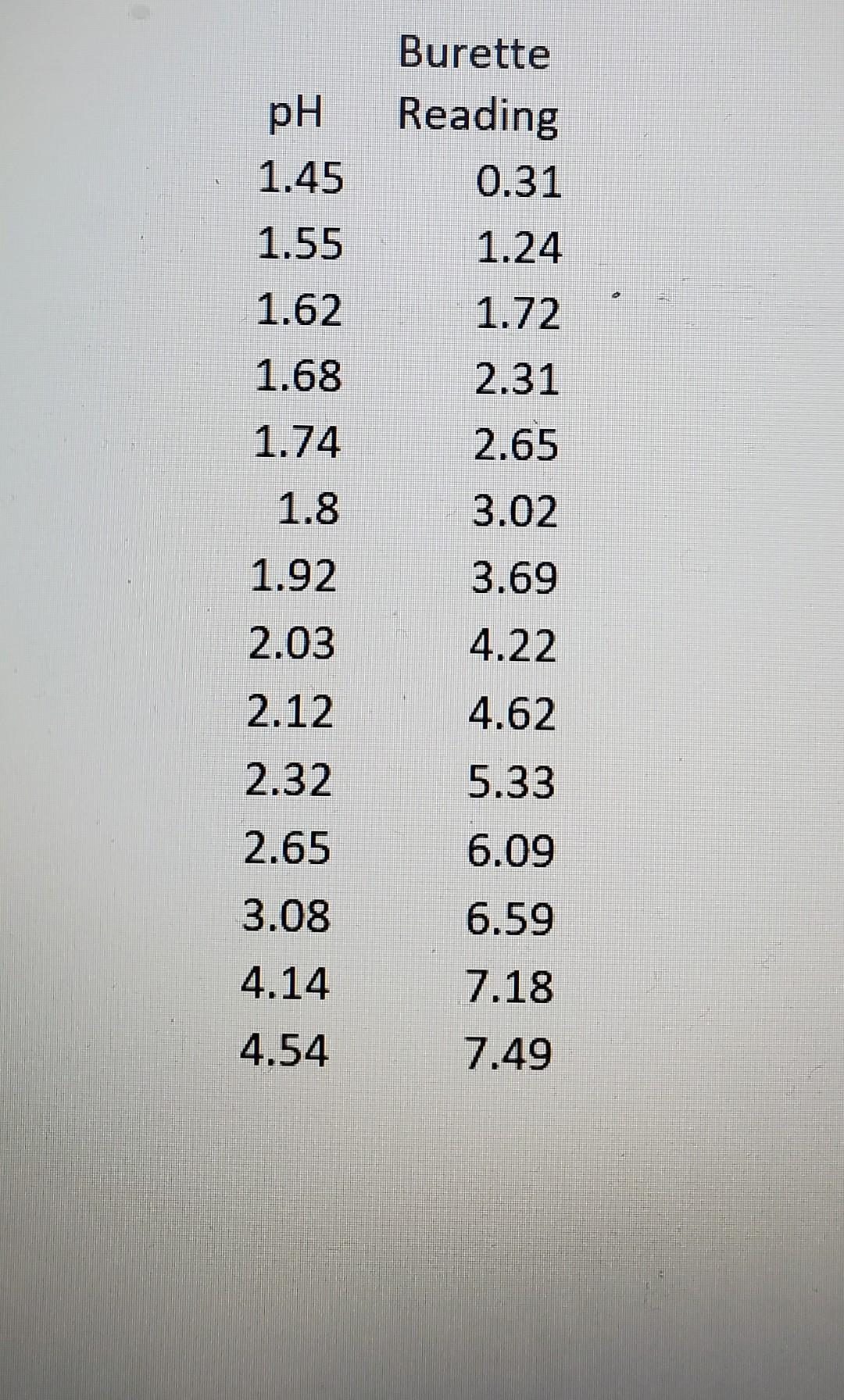
\begin{tabular}{cr} & Burette \\ pH & Reading \\ 1.45 & 0.31 \\ 1.55 & 1.24 \\ 1.62 & 1.72 \\ 1.68 & 2.31 \\ 1.74 & 2.65 \\ 1.8 & 3.02 \\ 1.92 & 3.69 \\ 2.03 & 4.22 \\ 2.12 & 4.62 \\ 2.32 & 5.33 \\ 2.65 & 6.09 \\ 3.08 & 6.59 \\ 4.14 & 7.18 \\ 4.54 & 7.49 \end{tabular}
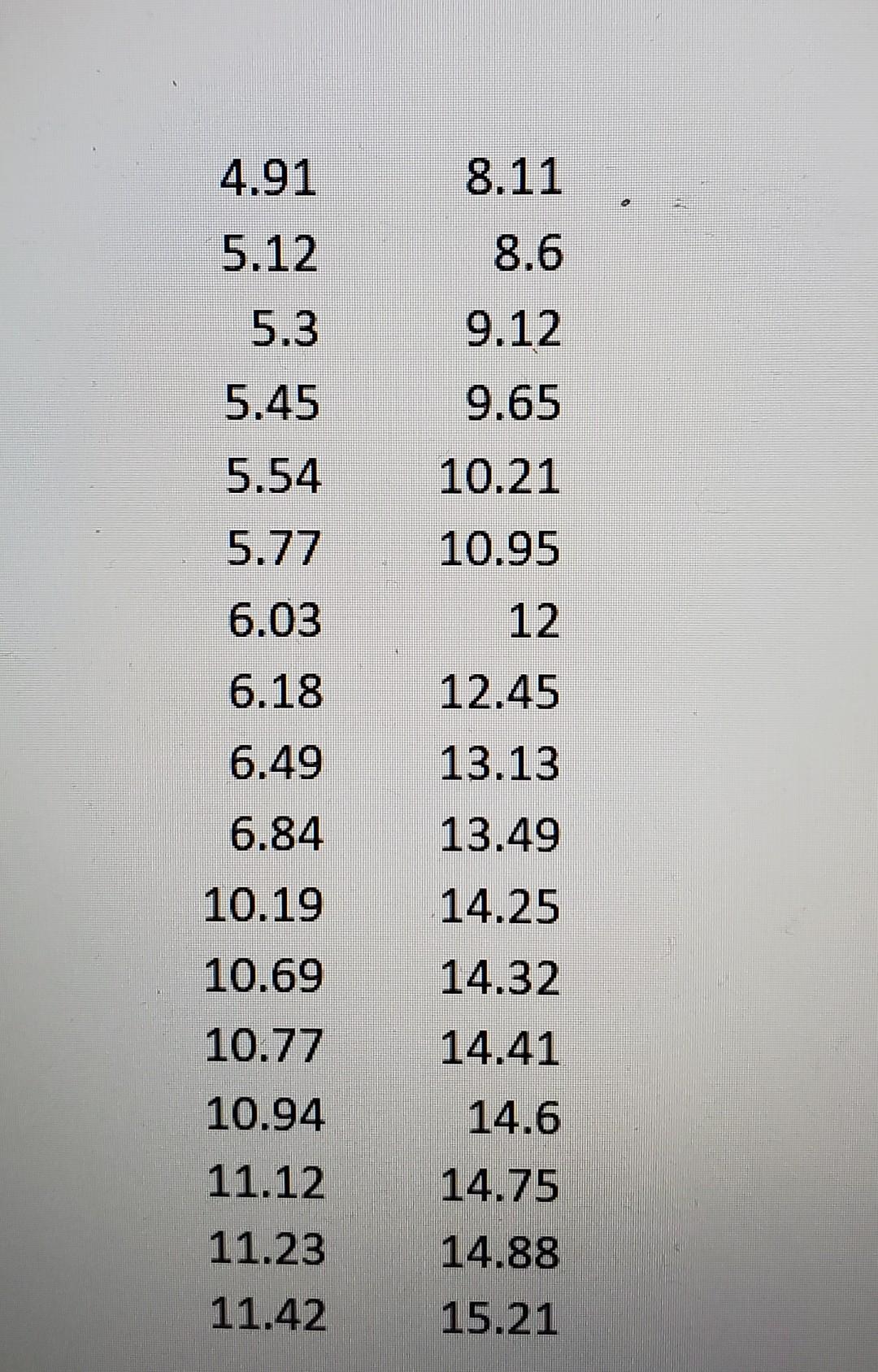
\begin{tabular}{rr} 4.91 & 8.11 \\ 5.12 & 8.6 \\ 5.3 & 9.12 \\ 5.45 & 9.65 \\ 5.54 & 10.21 \\ 5.77 & 10.95 \\ 6.03 & 12 \\ 6.18 & 12.45 \\ 6.49 & 13.13 \\ 6.84 & 13.49 \\ 10.19 & 14.25 \\ 10.69 & 14.32 \\ 10.77 & 14.41 \\ 10.94 & 14.6 \\ 11.12 & 14.75 \\ 11.23 & 14.88 \\ 11.42 & 15.21 \end{tabular}