Home /
Expert Answers /
Chemistry /
determine-the-ph-of-a-buffer-solution-by-constructing-an-ice-table-writing-the-equilibrium-constan-pa465
(Solved): Determine the pH of a buffer solution by constructing an ICE table, writing the equilibrium constan ...
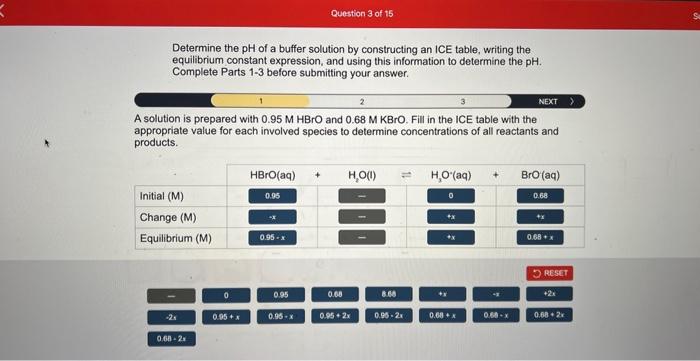
Determine the of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the . Complete Parts 1-3 before submitting your answer. A solution is prepared with and . Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products.
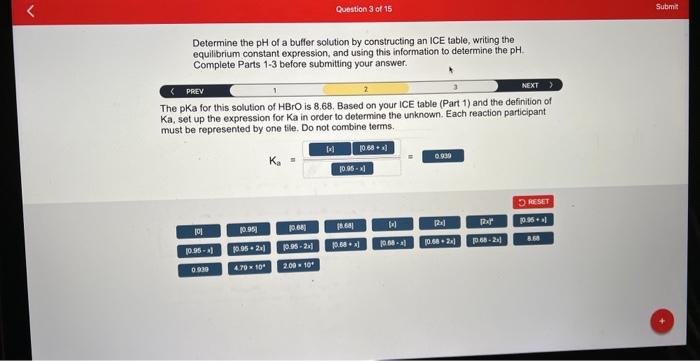
Determine the of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the Complete Parts 1-3 before submitting your answer. The pKa for this solution of is 8.68. Based on your ICE table (Part 1) and the definition of Ka, set up the expression for Ka in order to determine the unknown. Each reaction participant must be represented by one tile. Do not combine terms.
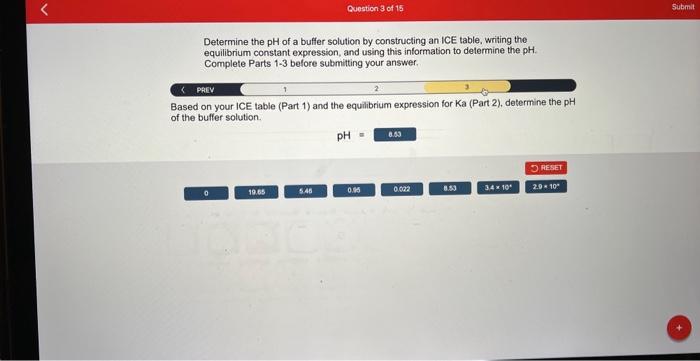
Determine the of a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and using this information to determine the . Complete Parts belore submitting your answer. PAEV 2 3 Based on your ICE table (Part 1) and the equilibrium expression for Ka (Part 2), determine the pH of the buffer solution.