Home /
Expert Answers /
Chemistry /
determine-the-moles-of-aici-produced-when-4-75-grams-of-cl-reacts-with-al-according-to-the-follo-pa485
(Solved): Determine the moles of AICI produced when 4.75 grams of Cl reacts with Al according to the follo ...
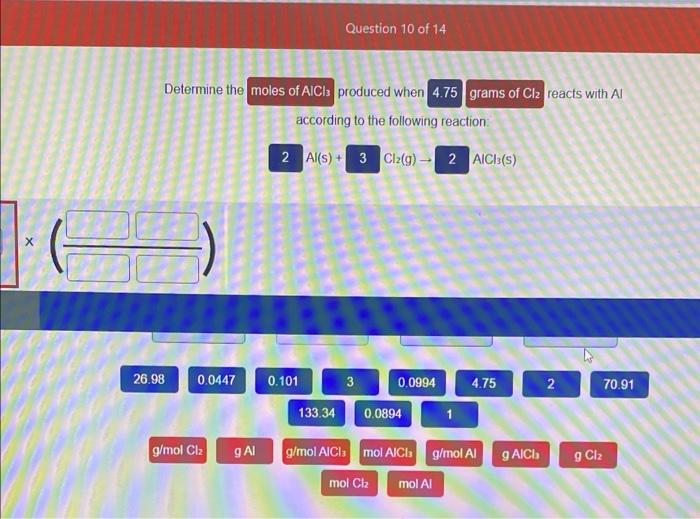
Determine the moles of AICI produced when 4.75 grams of Cl? reacts with Al according to the following reaction: 2 Al(s) + 3 Cl?(g) ? 2 AlCl³(s) 26.98 0.0447 g/mol Clz g Al Question 10 of 14 0.101 3 0.0994 4.75 133.34 g/mol AlCla mol AICI g/mol Al mol Clz mol Al 0.0894 g AlCh 2 g Clz 70.91
Expert Answer
2Al + 3Cl2 --> 2AlCl3 Mass of Cl2 = 4.75 g Molar mass of Cl2 = 70.91 g/mol