Home /
Expert Answers /
Chemistry /
determine-the-mass-of-co2-produced-by-burning-enough-of-methane-to-produce-2-50102kj-of-heat-pa974
(Solved): Determine the mass of CO2 produced by burning enough of methane to produce 2.50102kJ of heat ...
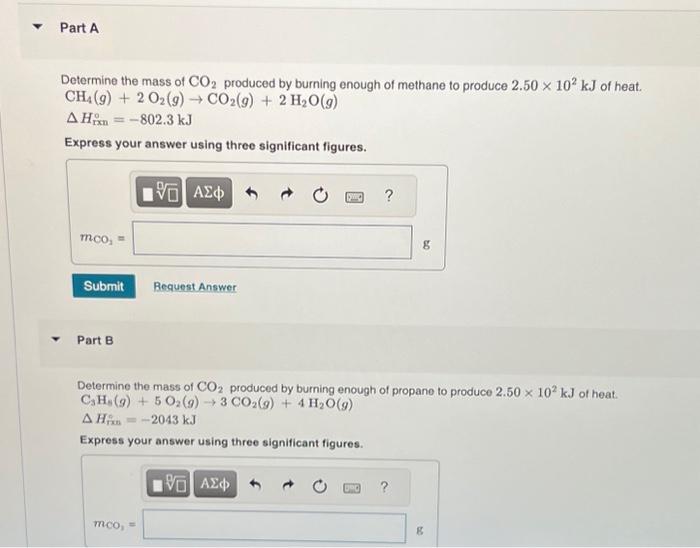

Determine the mass of produced by burning enough of methane to produce of heat. Express your answer using three significant figures. Part B Determine the mass of produced by burning enough of propane to produce of heat. Express your answer using three significant figures.
Determine the mass of produced by burning enough of octane to produce of heat. Express your answer using three significant figures.