Home /
Expert Answers /
Chemistry /
determine-the-mass-in-grams-of-co2-that-are-produced-by-the-complete-reaction-of-10-49-grams-of-pa204
(Solved): Determine the mass in grams of CO2 that are produced by the complete reaction of 10.49 grams of ...
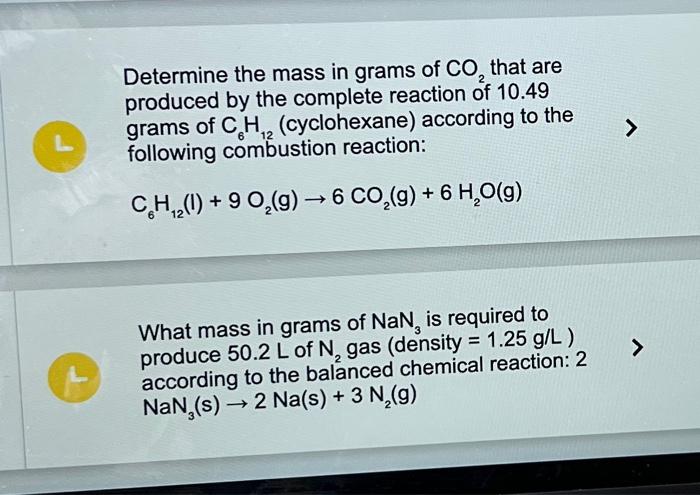
Determine the mass in grams of that are produced by the complete reaction of 10.49 grams of (cyclohexane) according to the following combustion reaction: What mass in grams of is required to produce of gas (density ) according to the balanced chemical reaction: 2