Home /
Expert Answers /
Chemistry /
determine-the-equilibrium-constant-for-the-formation-of-fescn-2-at-room-temperature-nbsp-please-s-pa491
(Solved): Determine the equilibrium constant for the formation of [FeSCN]2+ at room temperature. please s ...
Determine the equilibrium constant for the formation of [FeSCN]2+ at room temperature.
![MEASURED DATA
Part 1: Determining the Equilibrium Constant for the Formation of [FeSCN] at Room Temperature
A
Rxn Mix/Tube
1](https://media.cheggcdn.com/study/baf/baf0c357-729b-4955-b934-6cbfd7a4989a/image)
![A447
0.6
0.5
0.4
0.3
0.2
0.1
0 0
0.0E+00
2.0E-05
4.0E-05
6.0E-05
[FeSCN²+] (M)
8.0E-05
y = 4368.7x
1.0E-04
1.2E-04](https://media.cheggcdn.com/study/acf/acffff91-cbd3-488a-bb68-87f5984825db/image)
please show work answer all yellow highlighted portions calibration curve and data is provided
![MEASURED DATA
Part 1: Determining the Equilibrium Constant for the Formation of [FeSCN] at Room Temperature
A
Rxn Mix/Tube
1](https://media.cheggcdn.com/study/baf/baf0c357-729b-4955-b934-6cbfd7a4989a/image)

![A447
0.6
0.5
0.4
0.3
0.2
0.1
0 0
0.0E+00
2.0E-05
4.0E-05
6.0E-05
[FeSCN²+] (M)
8.0E-05
y = 4368.7x
1.0E-04
1.2E-04](https://media.cheggcdn.com/study/acf/acffff91-cbd3-488a-bb68-87f5984825db/image)
MEASURED DATA Part 1: Determining the Equilibrium Constant for the Formation of [FeSCN] at Room Temperature A Rxn Mix/Tube 1 2 3 4 5 Tube 6 (Mix 5, warm) 7 (Mix 5, cold) Rxn Mix/Tube Part 2: Observing the Effect of Temperature upon Ke C A447 512 1 2 F 3 f st 5 .307 211 528 A447 304 302 . 0 B 598 CALCULATED RESULTS Show work for at least one full set of data. Label calculations by their corresponding letter. Temperature, Rxn Mix 5 (°C) Part 1: Determining the Equilibrium Constant for the Formation of [FeSCN]** at Room Temperature. Use the calibration curve provided in your procedure to determine the [[FeSCN]2] at equilibrium. E Rxn 1 = [[FeSCN]2+]eg D Temperature (°C) 31-80 17.50 26.0°C - 37.8 ini-
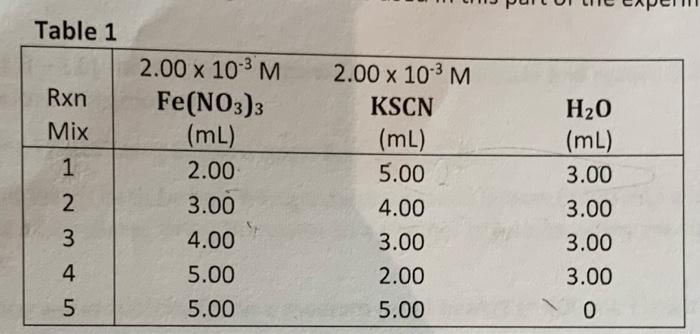
Table 1 Rxn Mix 12345 2.00 x 10-³ M Fe(NO3)3 (mL) 2.00 3.00 4.00 5.00 5.00 2.00 x 10-³ M KSCN (mL) 5.00 4.00 3.00 2.00 5.00 H?O (mL) 3.00 3.00 3.00 3.00 0
A447 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.0E+00 2.0E-05 4.0E-05 6.0E-05 [FeSCN²+] (M) 8.0E-05 y = 4368.7x 1.0E-04 1.2E-04
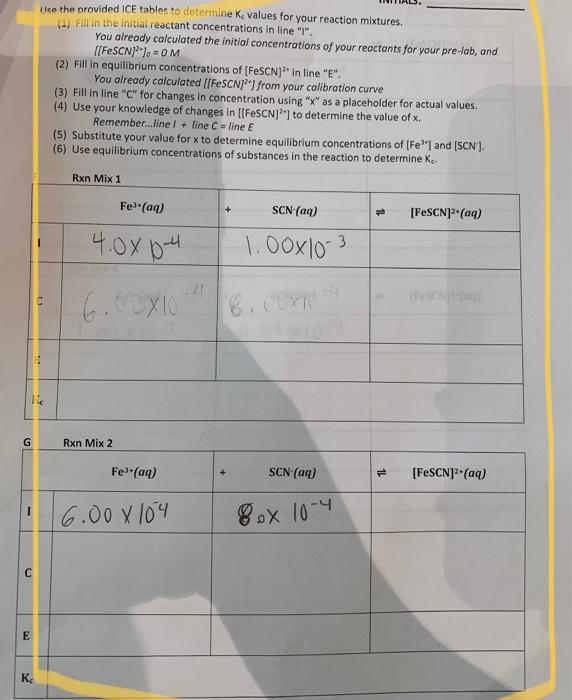
G C E K? Use the provided ICE tables to determine K, values for your reaction mixtures. (1) Fill in the initial reactant concentrations in line "I". You already calculated the initial concentrations of your reactants for your pre-lab, and [[FeSCN]"]=OM 11 (2) Fill in equilibrium concentrations of [FeSCN]2 in line "E". You already calculated [[FeSCN)] from your calibration curve (3) Fill in line "C" for changes in concentration using "x" as a placeholder for actual values. (4) Use your knowledge of changes in [[FeSCN]2] to determine the value of x. Remember...line !+ line C= line E (5) Substitute your value for x to determine equilibrium concentrations of [Fe³] and [SCN']. (6) Use equilibrium concentrations of substances in the reaction to determine K Rxn Mix 1 Fe³+ (aq) 4.0?0-4. 6.00×10 Rxn Mix 2 Fe³+ (aq) 6.00 x 104 .21 SCN- (aq) 1.00×10-3 8.00X10-9 SCN- (aq) 8ox 10-4 = [FeSCN]³+ (aq) [FeSCN]²+ (aq)