Home /
Expert Answers /
Chemistry /
determine-the-core-charge-for-a-magnesium-ion-mathrm-mg-2-and-use-it-as-an-approximati-pa420
(Solved): Determine the core charge for a magnesium ion, \( \mathrm{Mg}^{2+} \), and use it as an approximati ...
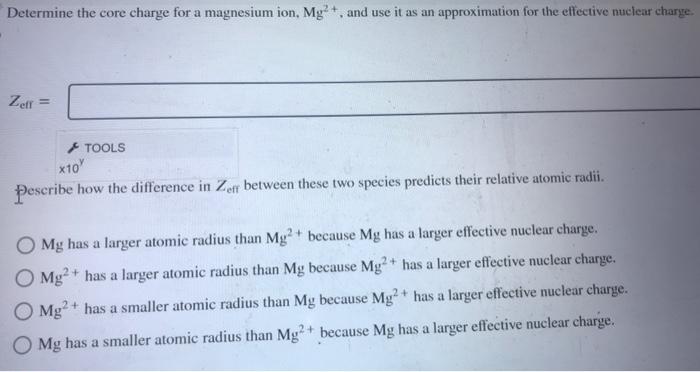
Determine the core charge for a magnesium ion, \( \mathrm{Mg}^{2+} \), and use it as an approximation for the effective nuclear charge. \( Z_{\mathrm{eff}}= \) Pescribe how the difference in \( Z_{\text {eff }} \) between these two species predicts their relative atomic radii. \( \mathrm{Mg} \) has a larger atomic radius than \( \mathrm{Mg}^{2}+ \) because \( \mathrm{Mg} \) has a larger effective nuclear charge. \( \mathrm{Mg}^{2}+ \) has a larger atomic radius than \( \mathrm{Mg} \) because \( \mathrm{Mg}^{2}+ \) has a larger effective nuclear charge. \( \mathrm{Mg}^{2}+ \) has a smaller atomic radius than \( \mathrm{Mg} \) because \( \mathrm{Mg}^{2}+ \) has a larger effective nuclear charge. \( \mathrm{Mg} \) has a smaller atomic radius than \( \mathrm{Mg}^{2}+ \) because \( \mathrm{Mg} \) has a larger effective nuclear charge.