Home /
Expert Answers /
Chemistry /
determine-the-concentration-of-c6h5nh3-in-a-buffer-solution-by-constructing-an-ice-table-pa137
(Solved): Determine the concentration of C6H5NH3+in a buffer solution by constructing an ICE table, ...
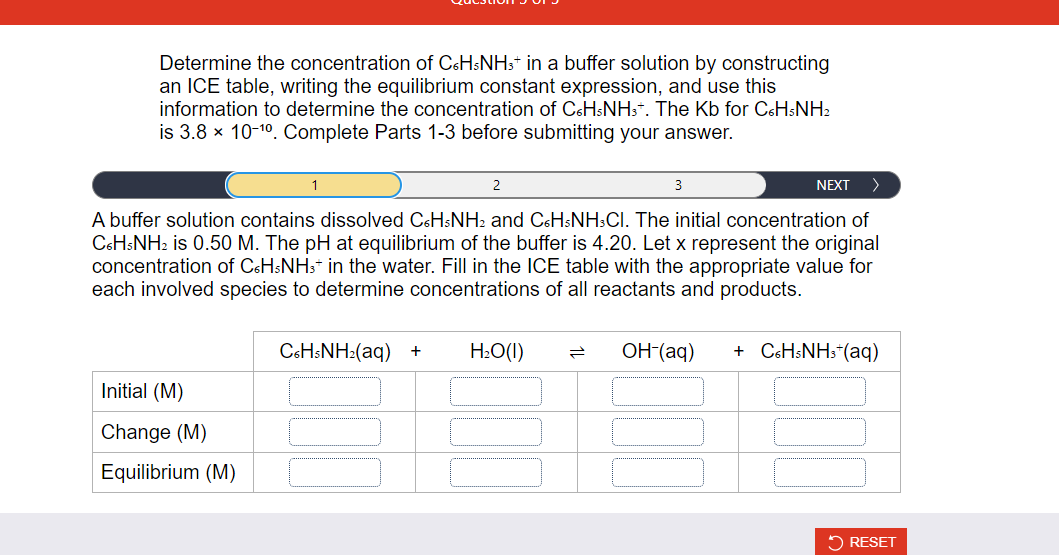
Determine the concentration of in a buffer solution by constructing an ICE table, writing the equilibrium constant expression, and use this information to determine the concentration of . The for is . Complete Parts before submitting your answer. A buffer solution contains dissolved and . The initial concentration of is . The at equilibrium of the buffer is 4.20 . Let represent the original concentration of in the water. Fill in the ICE table with the appropriate value for each involved species to determine concentrations of all reactants and products.