Home /
Expert Answers /
Chemistry /
determine-a-balanced-equation-for-the-reaction-of-molecular-nitrogen-n-and-oxygen-o-to-fo-pa393
(Solved): Determine a balanced equation for the reaction of molecular nitrogen (N) and oxygen (O) to fo ...
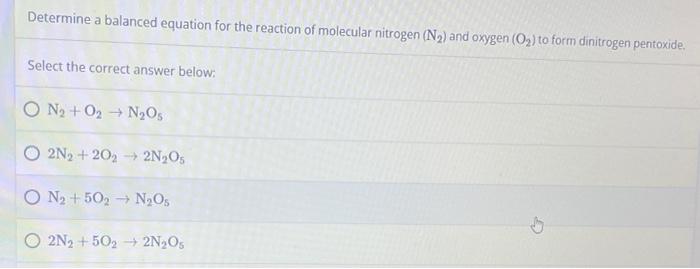
Determine a balanced equation for the reaction of molecular nitrogen (N?) and oxygen (O?) to form dinitrogen pentoxide. Select the correct answer below: ON?+O? N?O5 O2N? +202 ? 2N?Os O N? +502 ? N?Os 2N2 +502 2N?O5
Expert Answer
When N2 react with O2 to formed N2O5 we need to balanced between reactant and product. N2 and O2 both are reactants and N2O5 is produ