Home /
Expert Answers /
Chemistry /
determination-of-the-formula-of-a-hydrate-name-section-a-moles-of-anhydrous-salt-average-mass-pa675
(Solved): Determination of the Formula of a Hydrate Name: Section: A. Moles of anhydrous salt AVERAGE Mass ...


Determination of the Formula of a Hydrate Name: Section: A. Moles of anhydrous salt AVERAGE Mass of anhydrous salt: Formula of anhydrous salt Molar Mass of anhydrous salt: Mole of anhydrous salt CuSOy -SH?O B. Moles of water in hydrated salt AVERAGE Mass of water in hydrated salt :) Formula of water Molar Mass of water: Mole of water: C Formula of hydrated salt Mole Ratio: mole water/mole of anhydrous salt, Formula of hydrate: Name of hydrate: CALCULATIONS
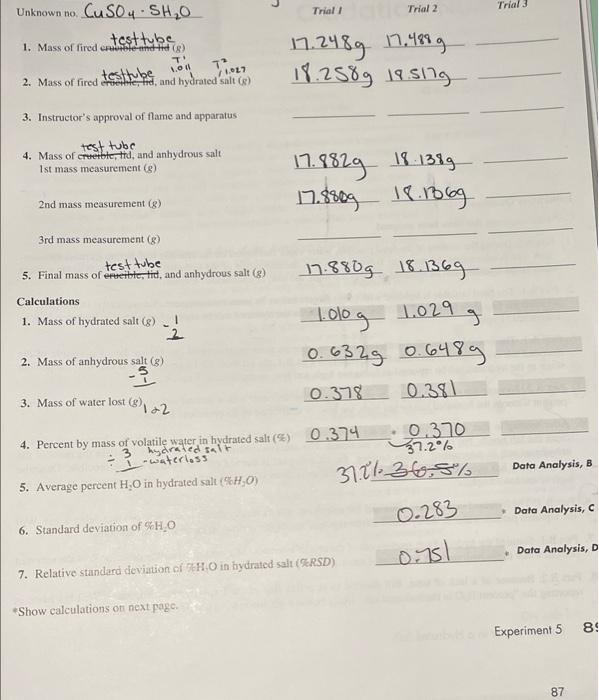
Unknown no. CuSO4 - SH?0 1. Mass of fired crucible-and-tid (g) test tube T' 2. Mass of fired ereble, id, and hydrated salt (g) testtube 1.00 71. /1.027 3. Instructor's approval of flame and apparatus test tube 4. Mass of creerble, tid, and anhydrous salt. 1st mass measurement (g) - 2nd mass measurement (g) 3rd mass measurement (g) test tube 5. Final mass of erweible, tid, and anhydrous salt (g) Calculations 1. Mass of hydrated salt (g)_ ! 2. Mass of anhydrous salt (g) -5 3. Mass of water lost (g) 2 4. Percent by mass of volatile water in hydrated salt (%) 0.374 hydrated salt 1-waterloss 5. Average percent H?O in hydrated salt (%H?O) 6. Standard deviation of %H?O 7. Relative standard deviation of H.O in hydrated salt (RSD) *Show calculations on next page. Trial I Trial 2 17.248g 17.488g 18.2589 18.5179 17.8829 18-1389 17.8809 18.1869 17.880g 18.1369 100g 1.029 g 0.6329 0.6489 0.378 0.381 0.370 37.2% 372136,5% 0-283 0751 Trial Data Analysis, B Data Analysis, C Data Analysis, D Experiment 5 8 87
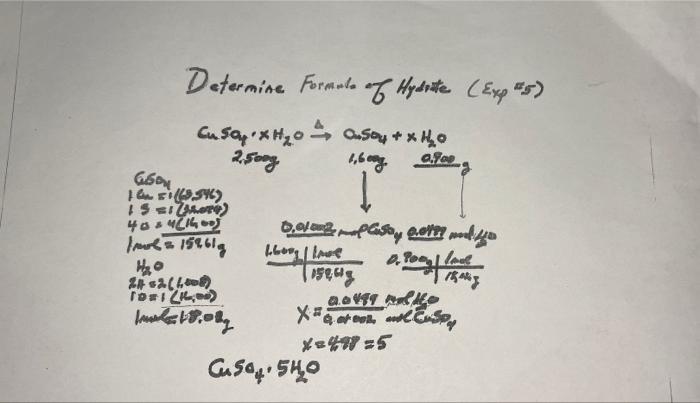
Determine Formule of Hydrite (Exp #5) + x1? Cu say x? 2.500g 0.900 1,600g I Csoy 0.0th and yo D. Pooled | 15mig 660 151 (3546) 15=1(0) 40=4 (1600) /mol = 152618 4?0 24 =2(1008) 10=1 (100) Im 18.08 0,01002 4600g | love 1/159.418 GOVET Xtook and Cusp X=498=5 Cuso 540
FORMULA OF HYDRATE CUSO4-X H20 (EXP # 5) A. Moles of anhydrous salt Avg mass of anhydrous salt 1.600 g Formula of anhydrous salt_CuSO4 Molar Mass of anhydrous salt_159.61 g Moles of anhydrous salt 0.01002 moles B. Moles of Water Avg mass of water _0.900 g Formula of water H2O Molar mass of water_18.02 g Moles of water 0.0499 moles C. Formula of Hydrated salt Mole ratio: Mol water/mol CuSO4 = 0.0499/0.01002=4.98 Formula of hydrate CuSO4-5H20 Name of hydrate: cupric sulfate pentahydrate