Home /
Expert Answers /
Chemistry /
details-and-explain-nbsp-55-mixing-aqueous-solutions-of-sodium-bicarbonate-and-calcium-chloride-re-pa225
(Solved): details and explain 55. Mixing aqueous solutions of sodium bicarbonate and calcium chloride re ...
details and explain
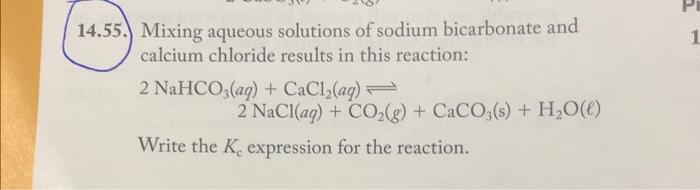
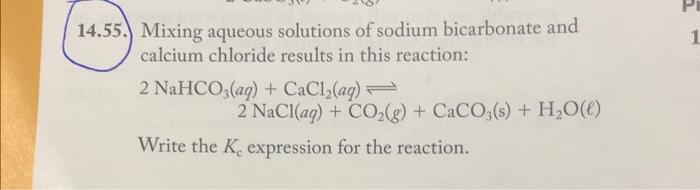
55. Mixing aqueous solutions of sodium bicarbonate and calcium chloride results in this reaction: \[ \begin{array}{l} 2 \mathrm{NaHCO}_{3}(a q)+\mathrm{CaCl}_{2}(a q) \rightleftharpoons \\ 2 \mathrm{NaCl}(a q)+\mathrm{CO}_{2}(g)+\mathrm{CaCO}_{3}(\mathrm{~s})+\mathrm{H}_{2} \mathrm{O}(\ell) \end{array} \] Write the \( K_{\mathrm{c}} \) expression for the reaction.
Expert Answer
2NaHCO3(aq) + CaCl2(aq) 2NaCl(aq) + CO2(g) + CaCO3(s) + H2O(l) Rate of forward reaction [Na