Home /
Expert Answers /
Chemistry /
derive-the-relationship-between-the-specific-heat-at-constant-volume-and-constant-pressure-for-an-id-pa888
(Solved): Derive the relationship between the specific heat at constant volume and constant pressure for an id ...
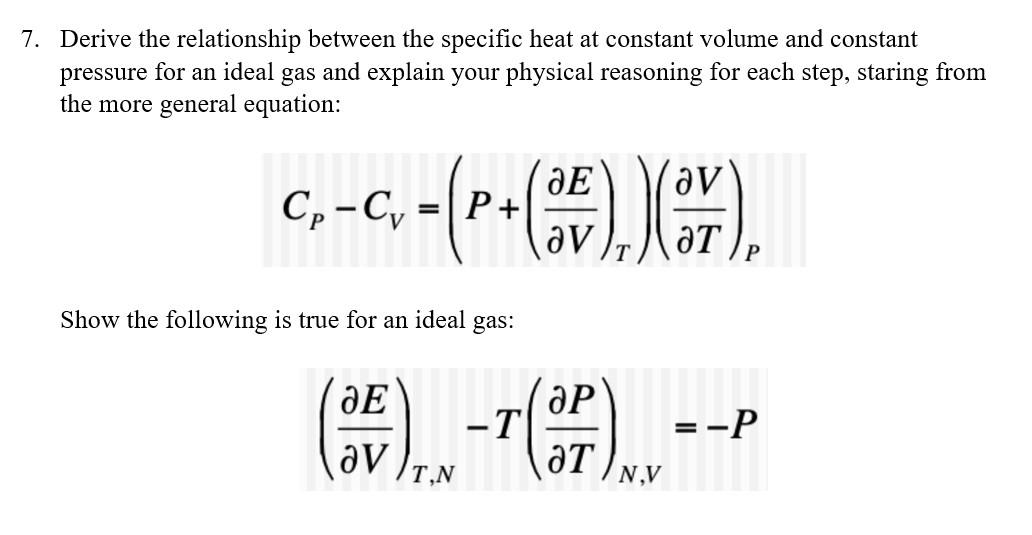
Derive the relationship between the specific heat at constant volume and constant pressure for an ideal gas and explain your physical reasoning for each step, staring from the more general equation:
Derive the relationship between the specific heat at constant volume and constant pressure for an ideal gas and explain your physical reasoning for each step, staring from the more general equation: \[ C_{P}-C_{V}=\left(P+\left(\frac{\partial E}{\partial V}\right)_{T}\right)\left(\frac{\partial V}{\partial T}\right)_{P} \] Show the following is true for an ideal gas: \[ \left(\frac{\partial E}{\partial V}\right)_{T, N}-T\left(\frac{\partial P}{\partial T}\right)_{N, V}=-P \]