Home /
Expert Answers /
Chemistry /
delta-h-for-the-reaction-mathrm-if-5-mathrm-g-rightarrow-mathrm-if-3-mathrm-pa121
(Solved): \( \Delta H \) for the reaction \( \mathrm{IF}_{5}(\mathrm{~g}) \rightarrow \mathrm{IF}_{3}(\mathrm ...
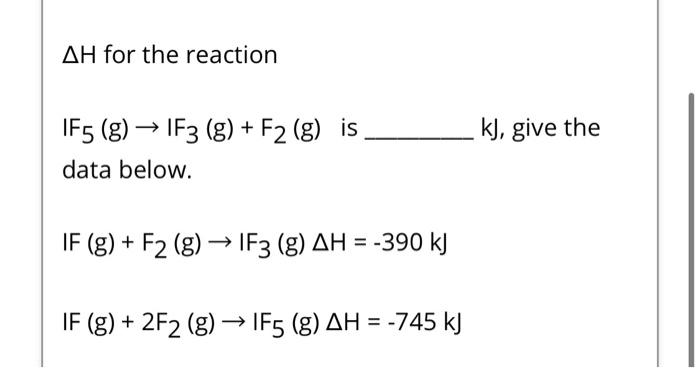
\( \Delta H \) for the reaction \( \mathrm{IF}_{5}(\mathrm{~g}) \rightarrow \mathrm{IF}_{3}(\mathrm{~g})+\mathrm{F}_{2}(\mathrm{~g}) \) is \( k J \), give the data below. \[ \mathrm{IF}(\mathrm{g})+\mathrm{F}_{2}(\mathrm{~g}) \rightarrow \mathrm{IF}_{3}(\mathrm{~g}) \Delta \mathrm{H}=-390 \mathrm{~kJ} \] \[ \mathrm{IF}(\mathrm{g})+2 \mathrm{~F}_{2}(\mathrm{~g}) \rightarrow \mathrm{IF}_{5}(\mathrm{~g}) \Delta \mathrm{H}=-745 \mathrm{~kJ} \]
Expert Answer
this question is completely based on Hess's Law. According