Home /
Expert Answers /
Chemical Engineering /
d-calculate-the-enthalpy-delta-mathrm-h-of-the-following-reaction-using-the-enthalpy-pa207
(Solved): d) Calculate the enthalpy \( (\Delta \mathrm{H}) \) of the following reaction using the enthalpy \ ...
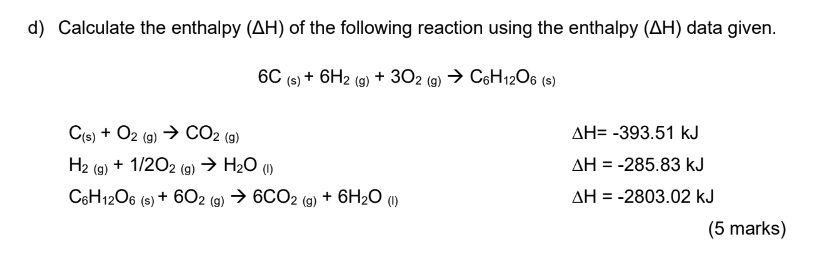
d) Calculate the enthalpy \( (\Delta \mathrm{H}) \) of the following reaction using the enthalpy \( (\Delta \mathrm{H}) \) data given. \[ 6 \mathrm{C}_{(\mathrm{s})}+6 \mathrm{H}_{2}(\mathrm{~g})+3 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6} \text { (s) } \] \[ \begin{array}{ll} \mathrm{C}_{(\mathrm{s})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{CO}_{2}(\mathrm{~g}) & \Delta \mathrm{H}=-393.51 \mathrm{~kJ} \\ \mathrm{H}_{2}(\mathrm{~g})+1 / 2 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{H}_{2} \mathrm{O}_{(\mathrm{l})} & \Delta \mathrm{H}=-285.83 \mathrm{~kJ} \\ \mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}(\mathrm{~s})+6 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 6 \mathrm{CO}_{2}(\mathrm{~g})+6 \mathrm{H}_{2} \mathrm{O}_{(\mathrm{l})} & \Delta \mathrm{H}=-2803.02 \mathrm{~kJ} \end{array} \] (5 marks)
Expert Answer
Here the answer is We have to multiply equation 1 and 2 by (6) So 6C + 6O2 ---