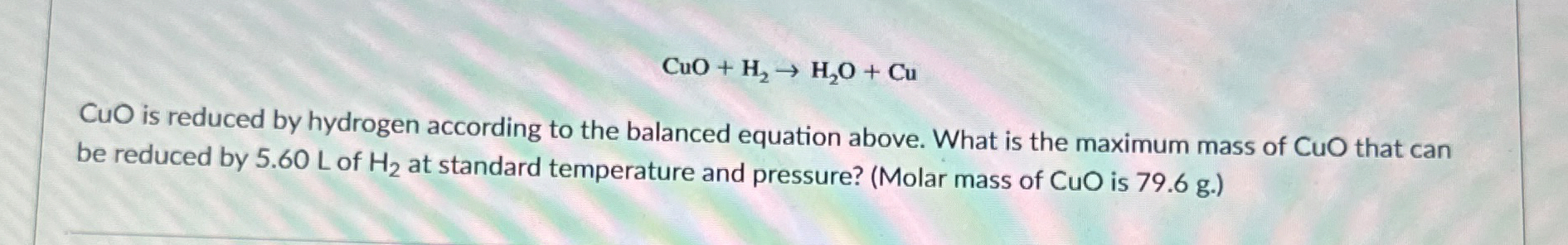Home /
Expert Answers /
Chemistry /
cuo-h-2-gt-h-2-o-cu-cuo-is-reduced-by-hydrogen-according-to-the-balanced-equation-above-what-is-pa623
(Solved): CuO+H_(2)->H_(2)O+Cu CuO is reduced by hydrogen according to the balanced equation above. What is ...
CuO+H_(2)->H_(2)O+Cu
CuOis reduced by hydrogen according to the balanced equation above. What is the maximum mass of
CuOthat can be reduced by 5.60 L of H2 at standard temperature and pressure? (molar mads of CuO is 79.6 g)