Home /
Expert Answers /
Chemistry /
create-a-plot-of-ecell-versus-log-1-ag-include-your-line-of-best-fit-and-r2-on-the-plot-proper-pa587
(Solved): Create a plot of Ecell versus log(1/[Ag+]). Include your line of best fit and R2 on the plot. Proper ...
Create a plot of Ecell versus log(1/[Ag+]). Include your line of best fit and R2 on the plot. Properly format the plot and include a descriptive figure caption below your figure. Explicitly write out how the line of best fit equation relates to the Nernst equation and your experimental Nernst equation. given the following data.
the concentration of [Ag+] is 0.1M
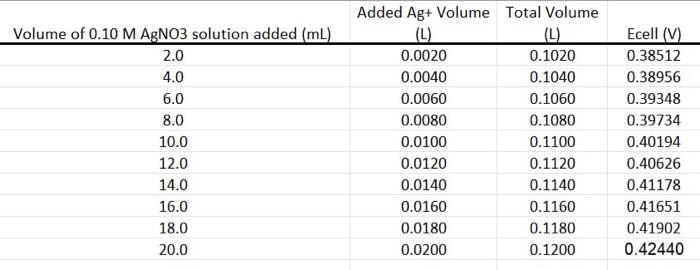
\begin{tabular}{|c|c|c|c|} \hline Volume of \( 0.10 \mathrm{M} \mathrm{AgNO3} \) solution added \( (\mathrm{mL}) \) & Added \( \mathrm{Ag}+ \) Volume \( (\mathrm{L}) \) & Total Volume \( (\mathrm{L}) \) & Ecell \( (\mathrm{V}) \) \\ \hline \( 2.0 \) & \( 0.0020 \) & \( 0.1020 \) & \( 0.38512 \) \\ \hline \( 4.0 \) & \( 0.0040 \) & \( 0.1040 \) & \( 0.38956 \) \\ \hline \( 6.0 \) & \( 0.0060 \) & \( 0.1060 \) & \( 0.39348 \) \\ \hline \( 8.0 \) & \( 0.0080 \) & \( 0.1080 \) & \( 0.39734 \) \\ \hline \( 10.0 \) & \( 0.0100 \) & \( 0.1100 \) & \( 0.40194 \) \\ \hline \( 12.0 \) & \( 0.0120 \) & \( 0.1120 \) & \( 0.40626 \) \\ \hline \( 14.0 \) & \( 0.0140 \) & \( 0.1140 \) & \( 0.41178 \) \\ \( 16.0 \) & \( 0.0160 \) & \( 0.1160 \) & \( 0.41651 \) \\ \hline \( 18.0 \) & \( 0.0180 \) & \( 0.1180 \) & \( 0.41902 \) \\ \hline \( 20.0 \) & \( 0.0200 \) & \( 0.1200 \) & \( 0.42440 \) \\ \hline \end{tabular}