Home /
Expert Answers /
Chemistry /
covalent-compounds-molecular-shapes-and-polarity-7-the-following-link-is-helpful-for-pa563
(Solved): Covalent Compounds: Molecular Shapes and Polarity 7 The following link is helpful for ...
Covalent Compounds: Molecular Shapes and Polarity












The following link is helpful for grasping molecular shape httns://ohet.colorado.edu/sims/htm//molecule-shapes/latest/molecule-shapes en.htm! To predict the shape of any molecule with a central atom following the octet rule: 1. Write the tewis electron dot structure of the molecule. This was discussed in Chapter 5 of your book. As a reminder, you will create the structure by carrying out the following steps in THE ORDER GIVEN: a. Add up all valence electrons in the molecule. - Example: in you have 4 valence electrons for the carbon atom, 1 valence electron for each hydrogen atom, and 6 valence electrons for the oxygen atom for a total of 12 valence electrons. b. Frame the structure: place the atom that tends to make the most bonds (the one with the most unpaired electrons in its Lewis symbol) at the center and arrange the other atoms around this central atom. Connect the center atom to each side atom with single bonds. - In , carbon has 4 unpaired electrons in its Lewis symbol, so it is placed in the middle and the hydrogen and oxygen atoms at the sides (see figure below). c. Fill in octets for the OUTER atoms, but NOT the central atom (yet!). - In the example of , the outer atoms are and . Remember that atoms only need 2 electrons to fill their valence shells, so the single bond to the atom is all they require. The atom has only the 2 electrons in the single bond to , and so needs 6 more atoms: d. Count the valence electrons used in the framed structure and subtract this number from the total valence electrons in the molecule (calculated in step a.) - In the example, each single bond in the framed structure represents 2 electrons; there are three single bonds electrons per bond, for a total of 6 valence electrons. Additionally, there are 3 lone pairs of electrons around the atom, for a total of 6 more electrons (each dot represents 1 electron). Therefore, 12 electrons have been used in this framed structure. 12 electrons in molecule -12 electrons used in framed structure electrons left
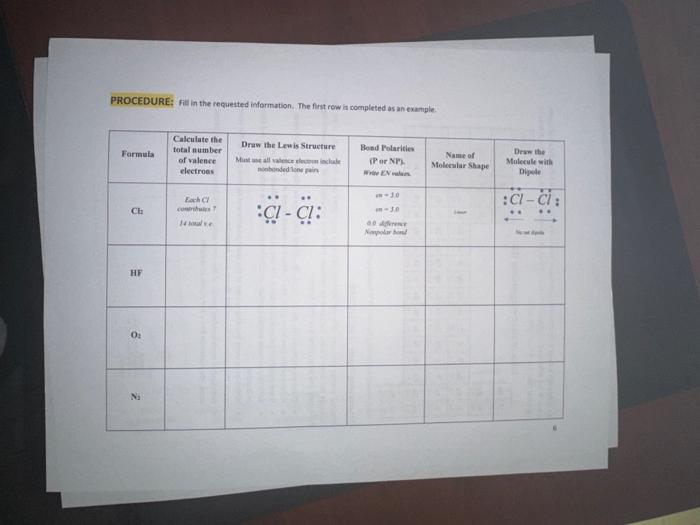
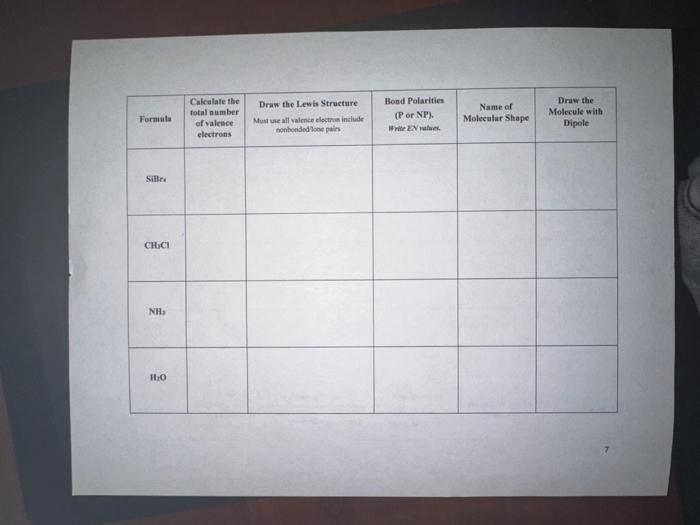
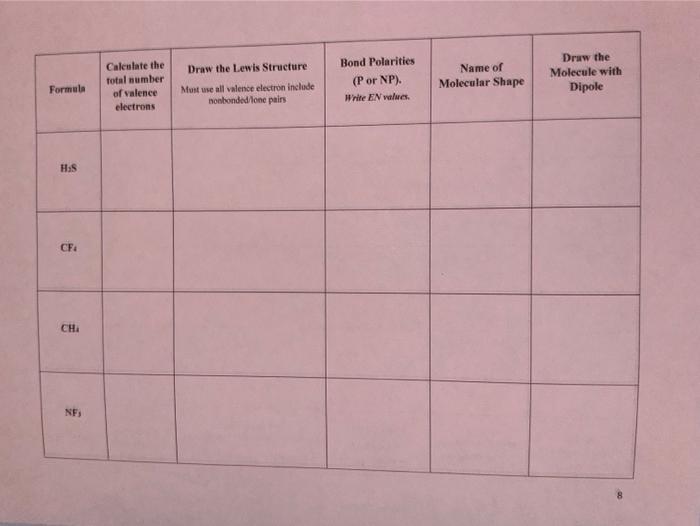
PROCEDURE: Fil in the requested information. The first row ia completed as an example.
the difference between the electronegativities of two atoms is less than 0.5 then the bond is nonpolar. If the difference is between 0.5 and 2.0 then the covalent bond is polar. If the differente is vreater than , it is an ionic bond. Rovel, introductory Chemistry, Ie, 02018 W. F feecman and Company Determining Dipole of MOLECULES: Unequal sharing between different atoms produces a polar bond or a net dipole. Once the molecule has been drawn, the covalent molecule itself may be nonpolar or polar depending on the nature and arrangement of their bonds: - Diatomic elements are aways no net dipole or is nonpolor. - If none of the bonds are polar, the molecule is no net dipole or is nonpoler. - If the molecule has one polar bond, the molecule has a net dipole or is polar. - With two or more bonding groups of different polarities, the molecule has a net dipole or is polar. - With two or more bonding groups of the same polarity and no lone pairs on the central atom, the molecule has no net dipole or is nonpolar. - With two or more bonding groups of the same polarity AND lone pairs on the central atom, the molecule hos a net dipole or is polar.
e. If there are any remaining valence electrons after the subtraction in step d, place these on the central atom (as palred electrons). - In this example, there were no valence electrons remaining (all the avallable valence electrons had been used), so no more electrons can be placed in the structure. f. Now look at the central atom to see if it has an octet. If it does not, REMEMBER THAT ALL. VALENCE ELECTRONS HAVE BEEN USED, and no more may be added. To give the centra! atom an octet in this case, a lone pair on an outer atom is converted into an adcitional covalent bond (i.e., forms a double bond). Remember, only use a double bond if steps and e failed to give an octet to all atoms. Only use a tripie bond if after steps arid AND after creating double bonds, one or more atoms still lacks an octet. - In this example, the atom does NOT have an octet: it has 3 single bonds which represents 6 electrons. Two more are needed. The atoms have no lone pairs of electrons to share, but the atom does: one lone pair will be converted to form a second bond between and ; Follow these steps for and you will notice that carbon lacks an octet when all 16 valence electrons are used. The only way to satisfy the octet rule for is by using multiple covalent bonds: 2. Once you have the Lewis structure, continue with determining the shape: count the number of bonding groups (bonded atoms) and lone pairs around the central atom: a. 4 bonding groups: Tetrahedral b. 3 bonding groups and 1 lone pair: Trigonal Pyramidal c. 2 bonding groups and 1 or 2 lone pairs: Angular (bent) d. 2 bonding groups and no lone pairs: Linear e. Diatomic molecule (only 2 atoms present in the whole molecule): tinear Determining Polarity of BONDS: A covalent bond between two atoms may be polor or nonpolar. This depends on the electronegotivity of each atom involved in the bond. Electronegativity is the tendency of an atom to attract electrons. Electronegativity is measured using numerical values (see table below). If 4
Objectives - Demonstrate ability to develop Lewis Structures of molecules - Determine the molecular shape (geometry) of a ghen molecule based on Lewis Structure - Determine the polarity of covalent bonds within a molecule and the polarity of the overall molecule. Discussion information about covalent bonding and Lewis structures can be found in Chapter 9 of your textbook. There are supplemental videos on Camvas. Be sure to read your text/watch these videos before proceeding with this lab. COVALENT BONDS Molecular compounds normally involve nonmetals covalently bonded to other nonmetals or metalloids. A covalent bond consists of a pair of shared electrons between two atoms. (Recall the locations of nonmetals in the Periodic Table). How many covalent bonds do atoms of the various nonmetais commonly form in order to follow the octet rule? A hydrogen atom, with only one electron to share, forms one covalent bond and no more. Other nonmetals and metalloids tend to form as many covalent bonds as there are single (unpaired) electrons in the dot formula of the element. While this rule is not "set in stone," it can be used as a guideline when assembling Lewis electron dot structures of molecules (Table H.1). Table H.1 Typical Numbers of Covalent Bonds Formed by Nonmetals and Metalloids
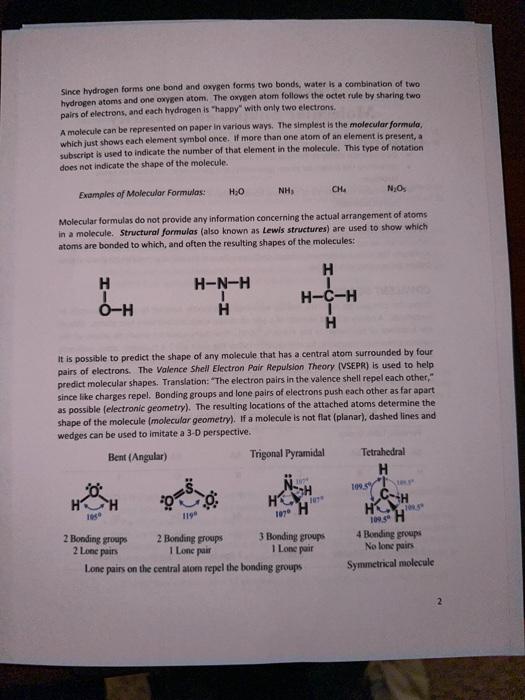
pairs of electrons, and each hydrogen is "happy" with only two electrons. A molecule can be represented on paper in various ways. The simplest is the molecular formula, which just shows each element symbol once. If more than one atom of an element is present, a subscript is used to indicate the number of that element in the molecule. This type of notation does not indicate the shape of the molecule. Exampies of Moleculor Formulas: NHs Molecular formulas do not provide any information concerning the actual arrangement of atoms in a molecule. Structural formulas (also known as Lewis structures) are used to show which atoms are bonded to which, and often the resulting shapes of the molecules: It is possible to predict the shape of any molecule that has a central atom surrounded by four pairs of electrons. The Valence Shell Electron Pair Aepulsion Theory (VSEPR) is used to help predict molecular shapes. Translation: "The electron pairs in the valence shell repel each other," since like charges repel. Bonding groups and lone pairs of electrons push each other as far apart as possible (electronic geometry). The resulting locations of the attached atoms determine the shape of the molecule (molecular geometry). If a molecule is not flat (planar), dashed lines and wedges can be used to imitate a 3-D perspective.