Home /
Expert Answers /
Chemistry /
copper-has-two-naturally-occurring-isotopes-cu-63-69-17-part-a-62-93-mathrm-amu-and-pa472
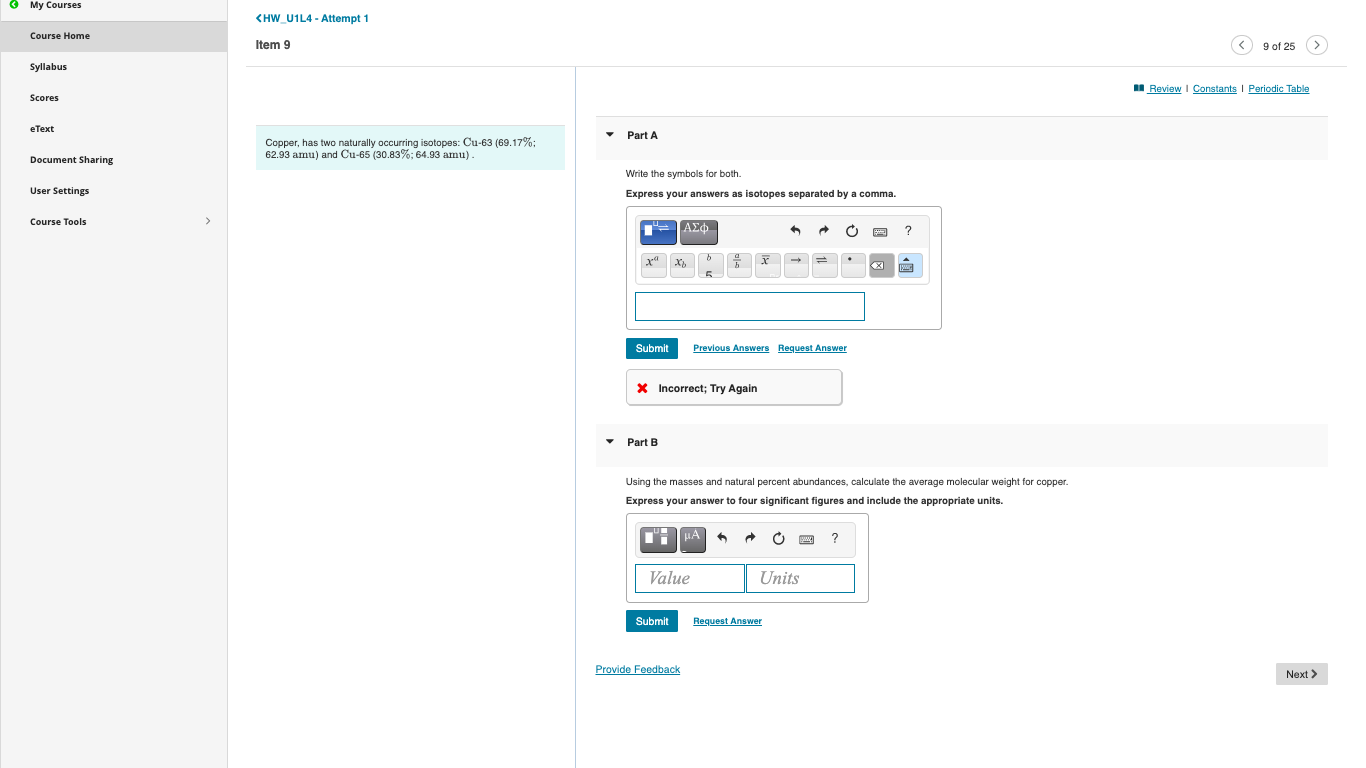
(Solved): Copper, has two naturally occurring isotopes: Cu-63 (69.17\%; Part A \( 62.93 \mathrm{amu}) \) and ...
Copper, has two naturally occurring isotopes: Cu-63 (69.17\%; Part A \( 62.93 \mathrm{amu}) \) and \( \mathrm{Cu}-65(30.83 \% ; 64.93 \mathrm{amu}) \). Write the symbols for both. Express your answers as isotopes separated by a comma. * Incorrect; Try Again Part B Using the masses and natural percent abundances, calculate the average molecular weight for copper. Express your answer to four significant figures and include the appropriate units.