Home /
Expert Answers /
Chemistry /
consider-this-three-step-mechanism-for-a-reaction-hint-check-the-energy-diagram-above-you-have-pa748
(Solved): Consider this three-step mechanism for a reaction (Hint: Check the energy diagram above-You have ...
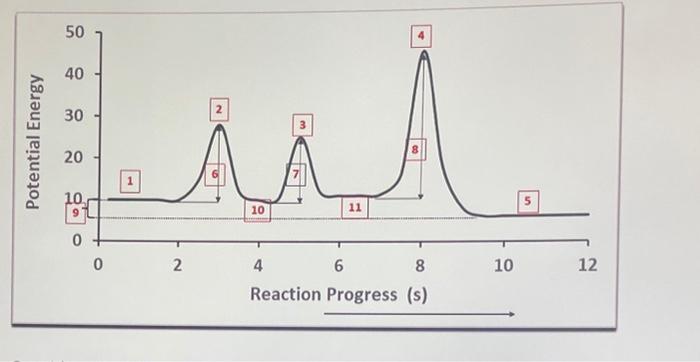
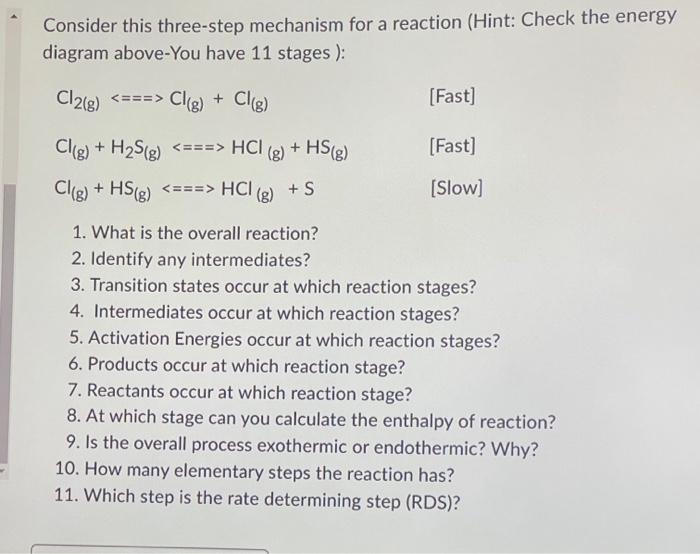
Consider this three-step mechanism for a reaction (Hint: Check the energy diagram above-You have 11 stages ): [Fast] [Fast] [Slow] 1. What is the overall reaction? 2. Identify any intermediates? 3. Transition states occur at which reaction stages? 4. Intermediates occur at which reaction stages? 5. Activation Energies occur at which reaction stages? 6. Products occur at which reaction stage? 7. Reactants occur at which reaction stage? 8. At which stage can you calculate the enthalpy of reaction? 9. Is the overall process exothermic or endothermic? Why? 10. How many elementary steps the reaction has? 11. Which step is the rate determining step (RDS)?