Home /
Expert Answers /
Chemistry /
consider-the-titration-of-30-0-mathrm-ml-of-0-0700-underline-mathrm-m-mathrm-nh-pa714
(Solved): Consider the titration of \( 30.0 \mathrm{~mL} \) of \( 0.0700 \underline{\mathrm{M}} \mathrm{NH}_ ...
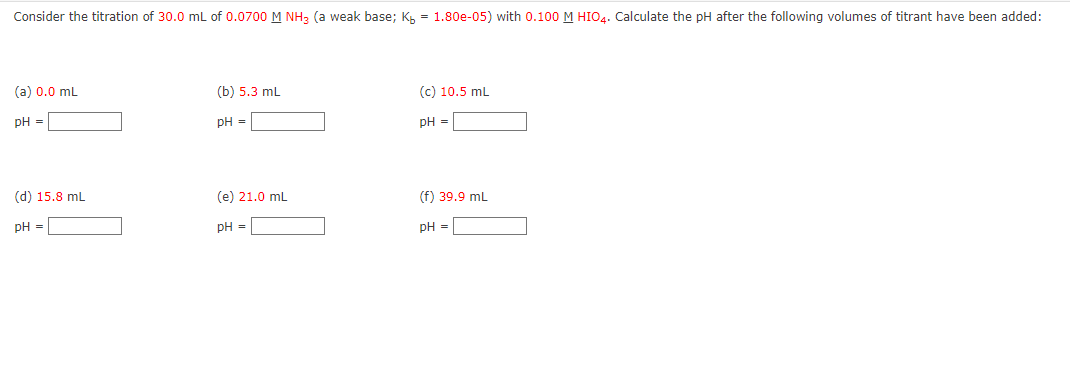
Consider the titration of \( 30.0 \mathrm{~mL} \) of \( 0.0700 \underline{\mathrm{M}} \mathrm{NH}_{3} \) (a weak base; \( \mathrm{K}_{\mathrm{b}}=1.80 \mathrm{e}-05 \) ) with \( 0.100 \underline{\mathrm{M}} \mathrm{HIO}{ }_{4} \). Calculate the pH after the following volumes of titrant have been added: (a) \( 0.0 \mathrm{~mL} \) (b) \( 5.3 \mathrm{~mL} \) (c) \( 10.5 \mathrm{~mL} \) \( \mathrm{pH}= \) \( \mathrm{pH}= \) \( \mathrm{pH}= \) (d) \( 15.8 \mathrm{~mL} \) (e) \( 21.0 \mathrm{~mL} \) (f) \( 39.9 \mathrm{~mL} \) \( \mathrm{pH}= \) \( \mathrm{pH}= \) pH \( = \)