Home /
Expert Answers /
Chemical Engineering /
consider-the-temperature-energy-scale-for-an-unknown-pure-substance-mathrm-mw-120-mathrm-g-pa677
(Solved): Consider the temperature energy scale for an unknown pure substance \( (\mathrm{MW}=120 \mathrm{~g ...
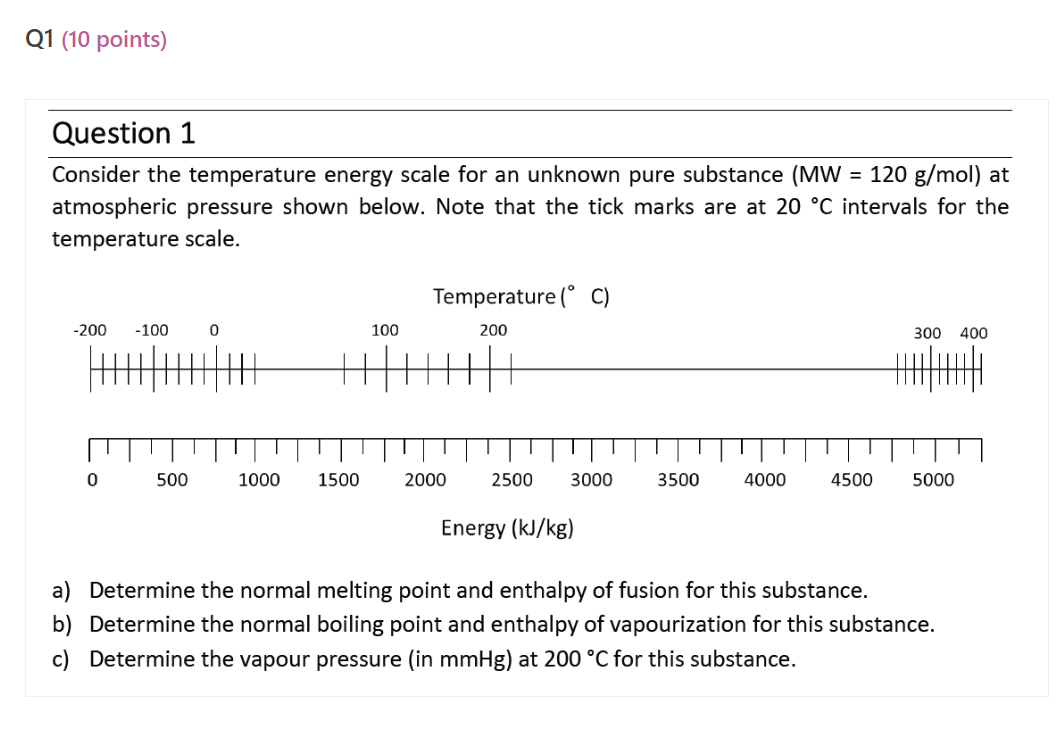
Consider the temperature energy scale for an unknown pure substance \( (\mathrm{MW}=120 \mathrm{~g} / \mathrm{mol}) \) at atmospheric pressure shown below. Note that the tick marks are at \( 20{ }^{\circ} \mathrm{C} \) intervals for the temperature scale. a) Determine the normal melting point and enthalpy of fusion for this substance. b) Determine the normal boiling point and enthalpy of vapourization for this substance. c) Determine the vapour pressure (in \( \mathrm{mmHg} \) ) at \( 200^{\circ} \mathrm{C} \) for this substance.
Expert Answer
Q. 1 Answer. A The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released o