Home /
Expert Answers /
Chemistry /
consider-the-reaction-of-so2-and-o2-described-by-the-chemical-reaction-below-determine-the-e-pa552
(Solved): Consider the reaction of SO2 and O2 described by the chemical reaction below. Determine the e ...
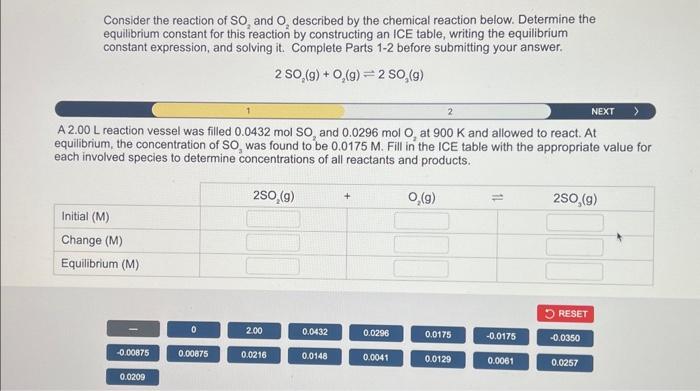
Consider the reaction of and described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts before submitting your answer. quilibrium, the concentration of was found to be . Fill in the ICE table with the appropriate value for ach involved species to determine concentrations of all reactants and products.
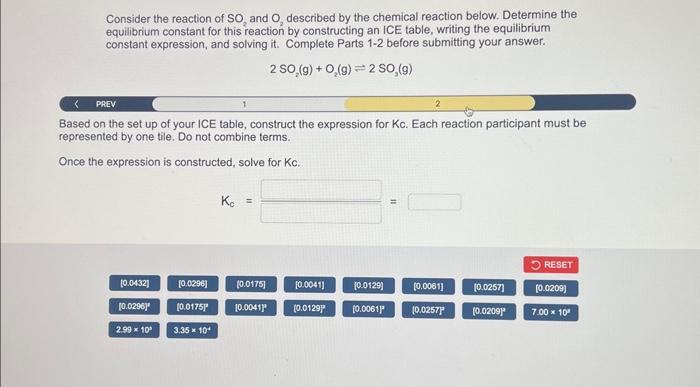
Consider the reaction of and described by the chemical reaction below. Determine the equilibrium constant for this reaction by constructing an ICE table, writing the equilibrium constant expression, and solving it. Complete Parts 1-2 before submitting your answer. PREV 1 2 Based on the set up of your ICE table, construct the expression for Kc. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for .
Expert Answer
Given,Initial moles of Initial moles of Volume Equilibrium concentration of Part aFill the ICE table for the given reaction-First, we have to calculate the initial concentrations of reactants and productsFormula used-Conce...