Home /
Expert Answers /
Chemistry /
consider-the-reaction-mathrm-c-2-mathrm-h-5-mathrm-cl-mathrm-g-mathrm-c-2-ma-pa142
(Solved): Consider the reaction \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl}(\mathrm{g})->\mathrm{C}_{2} \ma ...
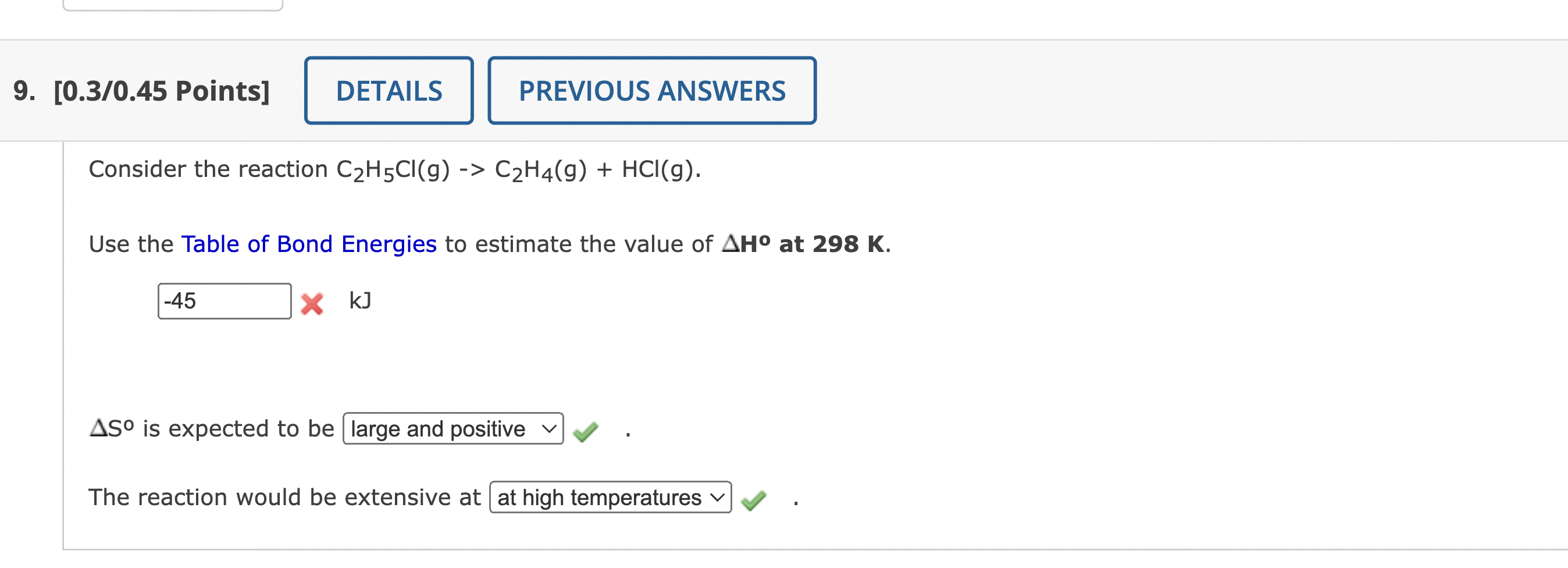
Consider the reaction \( \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{Cl}(\mathrm{g})->\mathrm{C}_{2} \mathrm{H}_{4}(\mathrm{~g})+\mathrm{HCl}(\mathrm{g}) \). Use the Table of Bond Energies to estimate the value of \( \Delta \mathbf{H}^{\circ} \) at \( 298 \mathrm{~K} \). \( x \mathrm{~kJ} \) \( \Delta \mathrm{S}^{0} \) is expected to be The reaction would be extensive at